Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A9OV
|
|||
| Former ID |
DCL000347
|
|||
| Drug Name |
TAK165
|
|||
| Synonyms |
Mubritinib; TAK 165; Mubritinib (USAN/INN); 4-[[4-[4-(triazol-1-yl)butyl]phenoxy]methyl]-2-[(E)-2-[4-(trifluoromethyl)phenyl]ethenyl]-1,3-oxazole
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Discontinued in Phase 1 | [1], [2] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Takeda Pharmaceuticals
|
|||
| Structure |
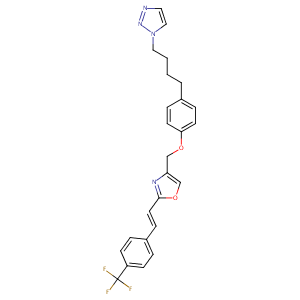 |
Download2D MOL |
||
| Formula |
C25H23F3N4O2
|
|||
| Canonical SMILES |
C1=CC(=CC=C1CCCCN2C=CN=N2)OCC3=COC(=N3)C=CC4=CC=C(C=C4)C(F)(F)F
|
|||
| InChI |
1S/C25H23F3N4O2/c26-25(27,28)21-9-4-20(5-10-21)8-13-24-30-22(18-34-24)17-33-23-11-6-19(7-12-23)3-1-2-15-32-16-14-29-31-32/h4-14,16,18H,1-3,15,17H2/b13-8+
|
|||
| InChIKey |
ZTFBIUXIQYRUNT-MDWZMJQESA-N
|
|||
| CAS Number |
CAS 366017-09-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
11969389, 14760763, 17398009, 43037052, 50490680, 57369821, 92721420, 99299526, 114571134, 123122738, 124360689, 124757654, 125164458, 126619164, 126661686, 131312231, 135178532, 135698690, 136348757, 136349478, 136367609, 137124306, 143157306, 144115322, 152057420, 152344431, 160682577, 162011934, 162037824, 162202684, 178102633, 179150389, 179322939, 186009055, 198947389, 204359629, 223396725, 223668772, 223704690, 223786982, 226472299, 241073514, 252157389, 252160688, 252215852, 252451421, 252543383
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6011). | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019257) | |||
| REF 3 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

