Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A6ZK
|
|||
| Former ID |
DNC014525
|
|||
| Drug Name |
2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one
|
|||
| Synonyms |
2,4,6-Cycloheptatrien-1-one, 2-hydroxy-3-(1-methylethyl)-; 1946-74-3; CHEMBL1275969; a-thujaplicine; thujaplicin; .alpha.-Thujaplicin; AC1Q6BZY; SCHEMBL355645; 2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one; AC1L2Z64; CTK1B5134; DTXSID70173098; TUFYVOCKVJOUIR-UHFFFAOYSA-N; ZINC2041733; BDBM50330793; AKOS025402371; AC-8489; LS-56188; 2-hydroxy-3-isopropyl-cyclohepta-2,4,6-trien-1-one; 2-hydroxy-3-propan-2-ylcyclohepta-2,4,6-trien-1-one; 2,4,6-Cycloheptatrien-1-one, 2-hydroxy(1-methylethyl)-; 38094-79-0
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
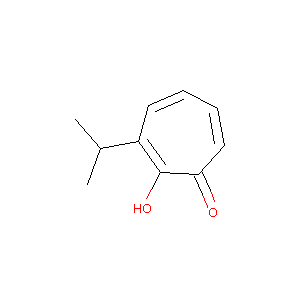 |
Download2D MOL |
||
| Formula |
C10H12O2
|
|||
| Canonical SMILES |
CC(C)C1=C(C(=O)C=CC=C1)O
|
|||
| InChI |
1S/C10H12O2/c1-7(2)8-5-3-4-6-9(11)10(8)12/h3-7H,1-2H3,(H,11,12)
|
|||
| InChIKey |
TUFYVOCKVJOUIR-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1946-74-3
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Tyrosinase (TYR) | Target Info | Inhibitor | [1] |
| BioCyc | (S)-reticuline biosynthesis | |||
| Eumelanin biosynthesis | ||||
| L-dopachrome biosynthesis | ||||
| KEGG Pathway | Tyrosine metabolism | |||
| Riboflavin metabolism | ||||
| Metabolic pathways | ||||
| Melanogenesis | ||||
| Pathwhiz Pathway | Riboflavin Metabolism | |||
| Tyrosine Metabolism | ||||
| WikiPathways | Dopamine metabolism | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Structural insights into the hot spot amino acid residues of mushroom tyrosinase for the bindings of thujaplicins. Bioorg Med Chem. 2010 Nov 15;18(22):8112-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

