Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A0UW
|
|||
| Former ID |
DNC004070
|
|||
| Drug Name |
6-Methoxy-5-oxazol-5-yl-2-phenyl-1H-indole
|
|||
| Synonyms |
CHEMBL23590; 569352-88-1; 6-Methoxy-5-oxazol-5-yl-2-phenyl-1H-indole; SCHEMBL5832036; CTK1F3437; DTXSID60623678; BDBM50126004; 2-Phenyl-5-(5-oxazolyl)-6-methoxy-1H-indole; 1H-Indole, 6-methoxy-5-(5-oxazolyl)-2-phenyl-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
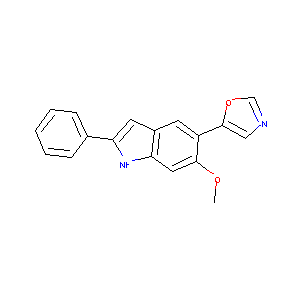 |
Download2D MOL |
||
| Formula |
C18H14N2O2
|
|||
| Canonical SMILES |
COC1=C(C=C2C=C(NC2=C1)C3=CC=CC=C3)C4=CN=CO4
|
|||
| InChI |
1S/C18H14N2O2/c1-21-17-9-16-13(7-14(17)18-10-19-11-22-18)8-15(20-16)12-5-3-2-4-6-12/h2-11,20H,1H3
|
|||
| InChIKey |
BESGGLGAKGKEOC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 569352-88-1
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Inosine-5'-monophosphate dehydrogenase 2 (IMPDH2) | Target Info | Inhibitor | [1] |
| BioCyc | Purine nucleotides degradation | |||
| Urate biosynthesis/inosine 5'-phosphate degradation | ||||
| Guanosine nucleotides de novo biosynthesis | ||||
| Superpathway of purine nucleotide salvage | ||||
| Purine nucleotides de novo biosynthesis | ||||
| Guanosine ribonucleotides de novo biosynthesis | ||||
| KEGG Pathway | Purine metabolism | |||
| Drug metabolism - other enzymes | ||||
| Metabolic pathways | ||||
| Panther Pathway | De novo purine biosynthesis | |||
| Reactome | Purine ribonucleoside monophosphate biosynthesis | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Novel indole-based inhibitors of IMPDH: introduction of hydrogen bond acceptors at indole C-3. Bioorg Med Chem Lett. 2003 Apr 7;13(7):1273-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

