Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A0FL
|
|||
| Former ID |
DAP001229
|
|||
| Drug Name |
Bupivacaine
|
|||
| Synonyms |
Anekain; Bloqueina; Bucaine; Bupivacaina; Bupivacainum; Bupivan; CBupivacaine; Carbostesin; DepoBupivacaine; Marcaina; Marcaine; Sensorcaine; Bupivacaine Carbonate; Bupivacaine HCL; Bupivacaine HCL KIT; Marcaine HCL; Marcaine Spinal; AH 250; Win 11318; Win 11318 HCl; Bucaine (TN); Bupivacaina [INN-Spanish]; Bupivacaine (INN); Bupivacaine Monohydrochloride, Monohydrate; Bupivacaine [INN:BAN]; Bupivacainum [INN-Latin]; DL-Bupivacaine; DUR-843; Marcain (TN); Marcaine (TN); Sensorcaine (TN); Sensorcaine-MPF; Sensorcaine-MPFSpinal; Transdur-Bupivacaine; Vivacaine (TN); Dl-1-Butyl-2',6'-pipecoloxylidide; (1)-1-Butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide; (inverted exclamation markA)-bupivacaine; 1-Butyl-2',6'-pipecoloxylidide; 1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide; 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Anaesthesia [ICD-11: 9A78.6; ICD-9: 338] | Approved | [1], [2] | |
| Pain [ICD-11: MG30-MG3Z] | Phase 2 | [3] | ||
| Therapeutic Class |
Anesthetics
|
|||
| Company |
AstraZeneca
|
|||
| Structure |
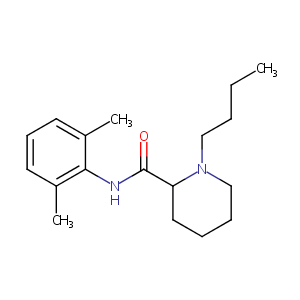 |
Download2D MOL |
||
| Formula |
C18H28N2O
|
|||
| Canonical SMILES |
CCCCN1CCCCC1C(=O)NC2=C(C=CC=C2C)C
|
|||
| InChI |
1S/C18H28N2O/c1-4-5-12-20-13-7-6-11-16(20)18(21)19-17-14(2)9-8-10-15(17)3/h8-10,16H,4-7,11-13H2,1-3H3,(H,19,21)
|
|||
| InChIKey |
LEBVLXFERQHONN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 38396-39-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9732, 4652641, 7978825, 8151651, 10513795, 11335933, 11361172, 11364418, 11366980, 11369542, 11371926, 11374654, 11377704, 11462144, 11466333, 11467453, 11485294, 11486225, 11489405, 11490789, 11492912, 11495338, 14897927, 29221638, 46506768, 47440286, 47736509, 47959774, 47959775, 48185025, 48259261, 48334521, 48415663, 48422999, 49698945, 49856308, 50010832, 50123233, 50123234, 51091874, 53788537, 57321340, 80394340, 81093368, 85209417, 85788472, 92309108, 92719756, 103068653, 103077120
|
|||
| ChEBI ID |
CHEBI:77431
|
|||
| ADReCS Drug ID | BADD_D00308 ; BADD_D00309 | |||
| SuperDrug ATC ID |
N01BB01
|
|||
| SuperDrug CAS ID |
cas=038396393
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Voltage-gated sodium channel alpha Nav1.8 (SCN10A) | Target Info | Modulator | [4] |
| Reactome | Interaction between L1 and Ankyrins | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2397). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 071810. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

