Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09ZXO
|
|||
| Former ID |
DNC000432
|
|||
| Drug Name |
Cilengitide
|
|||
| Synonyms |
Cilengitide; 188968-51-6; EMD-121974; Cilengitide [USAN:INN]; UNII-4EDF46E4GI; EMD121974; EMD-12192; EMD 121974; 4EDF46E4GI; CHEMBL429876; 2-[(2S,5R,8S,11S)-5-benzyl-11-[3-(diaminomethylideneamino)propyl]-7-methyl-3,6,9,12,15-pentaoxo-8-propan-2-yl-1,4,7,10,13-pentazacyclopentadec-2-yl]acetic acid; Cyclo(L-arginylglycyl-L-aspartyl-D-phenylalanyl-N-methyl-L-valyl); Cyclo(L-arginylglycyl-L-alpha-aspartyl-D-phenylalanyl-N-methyl-L-valyl); Cilengitide (TFA salt)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Rheumatoid arthritis [ICD-11: FA20] | Phase 3 | [1], [2] | |
| Structure |
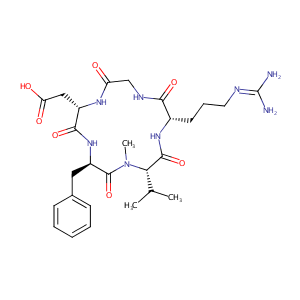 |
Download2D MOL |
||
| Formula |
C27H40N8O7
|
|||
| Canonical SMILES |
CC(C)C1C(=O)NC(C(=O)NCC(=O)NC(C(=O)NC(C(=O)N1C)CC2=CC=CC=C2)CC(=O)O)CCCN=C(N)N
|
|||
| InChI |
1S/C27H40N8O7/c1-15(2)22-25(41)33-17(10-7-11-30-27(28)29)23(39)31-14-20(36)32-18(13-21(37)38)24(40)34-19(26(42)35(22)3)12-16-8-5-4-6-9-16/h4-6,8-9,15,17-19,22H,7,10-14H2,1-3H3,(H,31,39)(H,32,36)(H,33,41)(H,34,40)(H,37,38)(H4,28,29,30)/t17-,18-,19+,22-/m0/s1
|
|||
| InChIKey |
AMLYAMJWYAIXIA-VWNVYAMZSA-N
|
|||
| CAS Number |
CAS 188968-51-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
825381, 12015138, 14764661, 14886950, 17397633, 33500423, 49956880, 50950930, 53790369, 57288492, 57288711, 57395325, 103570373, 104056223, 104425945, 126648372, 126666747, 134339858, 135156087, 137156914, 137262614, 162220925, 162808281, 163621005, 163686336, 164178308, 178103210, 179150114, 184823749, 198962979, 219812641, 223662751, 226088004, 226420604, 243159770, 249582596, 249583895, 252215145, 252471106
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6597). | |||
| REF 2 | ClinicalTrials.gov (NCT00689221) Cilengitide, Temozolomide, and Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma and Methylated Gene Promoter Status. U.S. National Institutes of Health. | |||
| REF 3 | The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells.PLoS One.2009;4(2):e4449. | |||
| REF 4 | Pharmacological inhibition of integrin alphavbeta3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis.Hepatology.2009 Nov;50(5):1501-11. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

