Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09RHQ
|
|||
| Former ID |
DAP001230
|
|||
| Drug Name |
Ropivacaine
|
|||
| Synonyms |
Naropin; ROPIVACAINE HCl; Ropivacaine hydrochloride; Ropivacaine monohydrochloride; Naropin (TN); Ropivacaine monohydrochloride, (S)-isomer; (2S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide; (2S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide hydrochloride
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Anaesthesia [ICD-11: 9A78.6; ICD-9: 338] | Approved | [1], [2] | |
| Therapeutic Class |
Anesthetics
|
|||
| Company |
AstraZeneca
|
|||
| Structure |
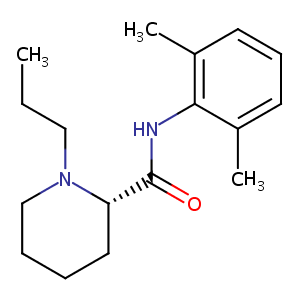 |
Download2D MOL |
||
| Formula |
C17H26N2O
|
|||
| Canonical SMILES |
CCCN1CCCCC1C(=O)NC2=C(C=CC=C2C)C
|
|||
| InChI |
1S/C17H26N2O/c1-4-11-19-12-6-5-10-15(19)17(20)18-16-13(2)8-7-9-14(16)3/h7-9,15H,4-6,10-12H2,1-3H3,(H,18,20)/t15-/m0/s1
|
|||
| InChIKey |
ZKMNUMMKYBVTFN-HNNXBMFYSA-N
|
|||
| CAS Number |
CAS 84057-95-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9735, 7980538, 10258266, 14775095, 14799444, 26758019, 29203717, 33499545, 46504712, 57395234, 75873274, 91746030, 93166361, 96025176, 103743807, 104425084, 124403617, 129915887, 131327602, 137260968, 143315334, 160963644, 162179066, 172914458, 175266235, 179151279, 184545821, 184685755, 198991501, 212342552, 223365937, 223533434, 223904012, 226419989, 241147628, 251912273, 251916578, 252356499, 252390150, 252426878, 252453683
|
|||
| ChEBI ID |
CHEBI:8890
|
|||
| ADReCS Drug ID | BADD_D01968 ; BADD_D01969 ; BADD_D02416 | |||
| SuperDrug ATC ID |
N01BB09
|
|||
| SuperDrug CAS ID |
cas=038396393
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Voltage-gated sodium channel alpha Nav1.8 (SCN10A) | Target Info | Modulator | [3] |
| Reactome | Interaction between L1 and Ankyrins | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7602). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 078601. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

