Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09PQZ
|
|||
| Drug Name |
Acalabrutinib
|
|||
| Synonyms |
Calquence; UNII-I42748ELQW; I42748ELQW; Acalabrutinib [INN]; Acalabrutinib [USAN:INN]; Calquence (TN); Acalabrutinib(ACP196); Acalabrutinib (ACP-196); GTPL8912; Acalabrutinib (JAN/USAN/INN); SCHEMBL14637368; EX-A881; WDENQIQQYWYTPO-IBGZPJMESA-N; KS-000006AT; KS-000006AD; s8116; BDBM50175583; AKOS030526094; ZINC208774715; DB11703; CS-5356; DS-3326
Click to Show/Hide
|
|||
| Indication | Mantle cell lymphoma [ICD-11: 2A85.5; ICD-10: C83.1] | Approved | [1] | |
| Chronic lymphocytic leukaemia [ICD-11: 2A82.0; ICD-10: C83.0, C91.1] | Phase 3 | [2] | ||
| Company |
AstraZeneca/Acerta Pharma
|
|||
| Structure |
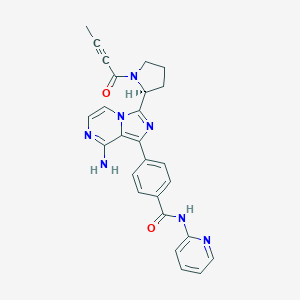 |
Download2D MOL |
||
| Formula |
C26H23N7O2
|
|||
| Canonical SMILES |
CC#CC(=O)N1CCCC1C2=NC(=C3N2C=CN=C3N)C4=CC=C(C=C4)C(=O)NC5=CC=CC=N5
|
|||
| InChI |
1S/C26H23N7O2/c1-2-6-21(34)32-15-5-7-19(32)25-31-22(23-24(27)29-14-16-33(23)25)17-9-11-18(12-10-17)26(35)30-20-8-3-4-13-28-20/h3-4,8-14,16,19H,5,7,15H2,1H3,(H2,27,29)(H,28,30,35)/t19-/m0/s1
|
|||
| InChIKey |
WDENQIQQYWYTPO-IBGZPJMESA-N
|
|||
| CAS Number |
CAS 1420477-60-6
|
|||
| PubChem Compound ID | ||||
| ADReCS Drug ID | BADD_D02482 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

