Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09HOW
|
|||
| Former ID |
DIB016165
|
|||
| Drug Name |
S-364735
|
|||
| Synonyms |
GSK-364735; HIV integrase inhibitors (2), Shionogi/GlaxoSmithKline
Click to Show/Hide
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Phase 2 | [1] | |
| Company |
Shionogi
|
|||
| Structure |
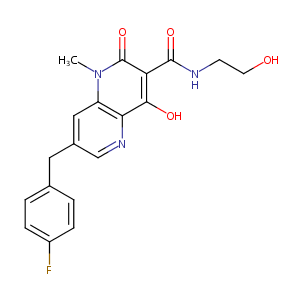 |
Download2D MOL |
||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Integrase (HIV IN) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00398125) Monotherapy Versus Placebo Over 10 Days in Integrase Naive HIV-1 Infected Adults. U.S. National Institutes of Health. | |||
| REF 2 | The naphthyridinone GSK364735 is a novel, potent human immunodeficiency virus type 1 integrase inhibitor and antiretroviral. Antimicrob Agents Chemother. 2008 Mar;52(3):901-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

