Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08YHS
|
|||
| Former ID |
DIB015014
|
|||
| Drug Name |
Linopirdine
|
|||
| Synonyms |
AVIVA; Linopirine; DuP-996
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Cognitive impairment [ICD-11: 6D71; ICD-10: F06.7] | Phase 3 | [1], [2] | |
| Company |
Bristol-Myers Squibb Pharma Co
|
|||
| Structure |
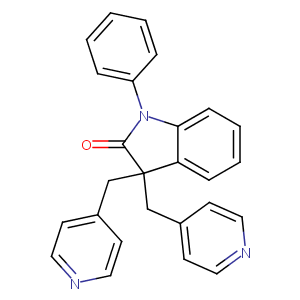 |
Download2D MOL |
||
| Formula |
C26H21N3O
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)N2C3=CC=CC=C3C(C2=O)(CC4=CC=NC=C4)CC5=CC=NC=C5
|
|||
| InChI |
1S/C26H21N3O/c30-25-26(18-20-10-14-27-15-11-20,19-21-12-16-28-17-13-21)23-8-4-5-9-24(23)29(25)22-6-2-1-3-7-22/h1-17H,18-19H2
|
|||
| InChIKey |
YEJCDKJIEMIWRQ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 105431-72-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
854025, 4659686, 7979778, 8152470, 11111362, 11114259, 12014138, 14927733, 17405293, 24263040, 24277846, 29223046, 46500471, 47206562, 48259468, 50070441, 50071084, 50104972, 50104973, 53777838, 57322057, 57654323, 77625157, 85177305, 85231103, 85788683, 90341523, 92304330, 103314113, 104304940, 121361490, 124749952, 124800387, 124880516, 124880517, 126407736, 126679061, 129783712, 135028219, 135650523, 137156939, 144203728, 162206962, 162224821, 163049819, 163564686, 170466342, 174006298, 178125900, 179149530
|
|||
| ChEBI ID |
CHEBI:34823
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Voltage-gated potassium channel Kv7.3 (KCNQ3) | Target Info | Modulator | [2] |
| KEGG Pathway | Cholinergic synapse | |||
| Reactome | Voltage gated Potassium channels | |||
| Interaction between L1 and Ankyrins | ||||
| WikiPathways | Potassium Channels | |||
| L1CAM interactions | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2599). | |||
| REF 2 | The M-channel blocker linopirdine is an agonist of the capsaicin receptor TRPV1. J Pharmacol Sci. 2010;114(3):332-40. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

