Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08RMH
|
|||
| Former ID |
DIB013841
|
|||
| Drug Name |
APC-2059
|
|||
| Synonyms |
BAY-44-3965
Click to Show/Hide
|
|||
| Indication | Inflammatory bowel disease [ICD-11: DD72; ICD-10: K50-K52] | Discontinued in Phase 2 | [1] | |
| Company |
Celera South San Francisco
|
|||
| Structure |
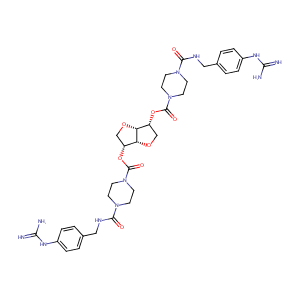 |
Download2D MOL |
||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Tryptase alpha/beta-1 (Tryptase) | Target Info | Inhibitor | [2] |
| Reactome | Activation of Matrix Metalloproteinases | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010270) | |||
| REF 2 | Treatment of mildly to moderately active ulcerative colitis with a tryptase inhibitor (APC 2059): an open-label pilot study. Aliment Pharmacol Ther. 2002 Mar;16(3):407-13. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

