Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08LDB
|
|||
| Former ID |
DCL000405
|
|||
| Drug Name |
GSK1265744
|
|||
| Synonyms |
Cabotegravir; 1051375-10-0; GSK1265744; UNII-HMH0132Z1Q; GSK744; GSK1265744A; GSK-1265744; HMH0132Z1Q; Cabotegravir (GSK744, GSK1265744); CAB; Oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide, N-[(2,4-difluorophenyl)methyl]-2,3,5,7,11,11a-hexahydro-6-hydroxy-3-methyl-5,7-dioxo-, (3S,11aR)-;Oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide, N-[(2,4-difluorophenyl)methyl]-2,3,5,7,11,11a-hexahydro-6-hydroxy-3-methyl-5,7-dioxo-, (3S,11aR)-; Cabotegravir [USAN:INN]; GSK744 LAP; GSK744 LA; S/GSK1265744; GSK 744; 744 LA; GSK 1265
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62] | Phase 3 | [1] | |
| Company |
GSK
|
|||
| Structure |
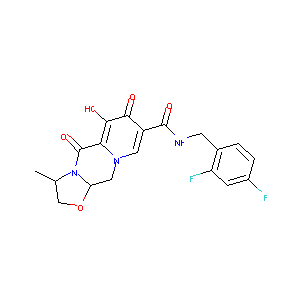 |
Download2D MOL |
||
| Formula |
C19H17F2N3O5
|
|||
| Canonical SMILES |
CC1COC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O
|
|||
| InChI |
1S/C19H17F2N3O5/c1-9-8-29-14-7-23-6-12(16(25)17(26)15(23)19(28)24(9)14)18(27)22-5-10-2-3-11(20)4-13(10)21/h2-4,6,9,14,26H,5,7-8H2,1H3,(H,22,27)/t9-,14+/m0/s1
|
|||
| InChIKey |
WCWSTNLSLKSJPK-LKFCYVNXSA-N
|
|||
| CAS Number |
CAS 1051375-10-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Integrase (HIV IN) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02951052) Study Evaluating the Efficacy, Safety, and Tolerability of Switching to Long-acting Cabotegravir Plus Long-acting Rilpivirine From Current Antiretroviral Regimen in Virologically Suppressed HIV-1-infected Adults. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

