Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08JCA
|
|||
| Former ID |
DAP001321
|
|||
| Drug Name |
Silver sulfadiazine
|
|||
| Synonyms |
Brandiazin; Dermazin; Dermazine; Flamazine; Flammazine; Geben; Sicazine; Silbertone; Silvederma; Silver; Sulfargen; Abbott Brand of Silver Sulfadiazine; Aldo Brand of Silver Sulfadiazine; Major Brand of Silver Sulfadiazine; Medphano Brand of Silver Sulfadiazine; MonarchBrand of Silver Sulfadiazine; Pharmascience Brand of Silver Sulfadiazine; Rhone Poulenc Rorer Brand of Silver Sulfadiazine; SSD AF; SULFADIAZINE SILVER; Sherwood Brand of Silver Sulfadiazine; Silver Sulfafdiazine; Silver sulfadiazinate; Silver sulphadiazine; Solvay Brand of Silver Sulfadiazine; Sulfadiazin silber; Sulfadiazine silver salt; Sulfadiazine silverSilvadene; Zenith Brand of Silver Sulfadiazine; Par Brand 1 of Silver Sulfadiazine; Par Brand 2 of Silver Sulfadiazine; Par Brand 3 of Silver Sulfadiazine; Flamazine (TN); Rhone-Poulenc Rorer Brand of Silver Sulfadiazine; Silvadene (TN); Silver(I) sulfadiazine; Smith & Nephew Brand of Silver Sulfadiazine; Sulfadiazin, silbersalz; Sulfadiazine silver (JP15); Sulfadiazine, Silver [USAN]; Sulfadiazine, silver; Sulfafdiazine, Silver; Thermazene (TN); SSD (1% Silver Sulfadiazine Cream USP); Sulfadiazine, silver (USP); N1-2-Pyrimidinylsulfanilamide monosilver(1+) salt; N(sup 1)-2-Pyrimidinylsulfanilamide monosilver(1+) salt; Silver (4-aminophenyl)sulfonyl-pyrimidin-2-ylazanide; Silver 4-amino-N-pyrimidin-2-ylbenzenesulfonamide; Benzenesulfonamide,4-amino-N-2-pyrimidinyl-, silver complex; Silver(1+) [(4-aminophenyl)sulfonyl](pyrimidin-2-yl)azanide; Sulfanilamide,N1-2-pyrimidinyl-, monosilver(1+) salt; Sulfanilamide, N(sup 1)-2-pyrimidinyl-, monosilver(1+) salt; Benzenesulfonamide, 4-amino-N-2-pyrimidinyl-, monosilver(1+) salt; (4-Amino-N-pyrimidin-2-ylbenzenesulphonamidato-NN,O1)silver; 4-Amino-N-(2-pyrimidinyl)benzenesulfonamide silver salt
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Bacterial infection [ICD-11: 1A00-1C4Z; ICD-10: A00-B99] | Approved | [1], [2], [3] | |
| Therapeutic Class |
Antiinfective Agents
|
|||
| Structure |
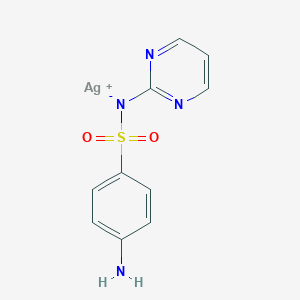 |
Download2D MOL
|
||
| Formula |
C10H9AgN4O2S
|
|||
| Canonical SMILES |
C1=CN=C(N=C1)[N-]S(=O)(=O)C2=CC=C(C=C2)N.[Ag+]
|
|||
| InChI |
1S/C10H9N4O2S.Ag/c11-8-2-4-9(5-3-8)17(15,16)14-10-12-6-1-7-13-10;/h1-7H,11H2;/q-1;+1
|
|||
| InChIKey |
UEJSSZHHYBHCEL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 22199-08-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:9142
|
|||
| ADReCS Drug ID | BADD_D02022 ; BADD_D02023 ; BADD_D02024 | |||
| SuperDrug ATC ID |
D06BA01; D08AL30; J01EC02
|
|||
| SuperDrug CAS ID |
cas=022199082
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial Dihydropteroate synthetase (Bact folP) | Target Info | Binder | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4126). | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 019608. | |||
| REF 4 | Comparative evaluation of transdermal formulations of norfloxacin with silver sulfadiazine cream, USP, for burn wound healing property. J Burns Wounds. 2006 Jun 7;5:e4. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

