Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08JAC
|
|||
| Former ID |
DIB003719
|
|||
| Drug Name |
Fenretinide
|
|||
| Synonyms |
RT-101; ST-602; SYT-101; Fenretinide (pulmonary Pseudomonas aeruginosa infections in cystic fibrosis), McGill University; LXS/4-HPR; Fenretinide (Ewing's sarcoma), Cancer Research UK; Fenretinide (ILE formulation), Children's Hospital LA; Fenretinide (intralipid emulsion formulation), Children's Hospital of Los Angeles; Fenretinide (intravenous, cancer), SciTech Development; Fenretinide (iv), Children's Hospital LA; Fenretinide (oral, AMD), Sytera; Fenretinide (soft gel capsule, AMD), ReVision Therapeutics; Fenretinide (soft gel capsule, AMD), Sirion; Fenretinide (topical, actinic keratosis and cancer), SciTech Development; N-(4-hydroxyphenyl)retinamide; Fenretinide (intravenous emulsion, lymphoma/solid tumors/pediatric neuroblastomas/pediatric leukemia), CerRx; Fenretinide (oral powder/Lym-X-Sorb, neuroblastoma), BioMolecular Products; 4-HPR
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Macular degeneration [ICD-11: 9B78.3; ICD-10: H35.3; ICD-9: 362.5] | Phase 3 | [1] | |
| Lymphoma [ICD-11: 2A80-2A86; ICD-9: 202.8, 208.9] | Phase 2 | [2] | ||
| Peripheral T-cell lymphoma [ICD-11: 2A90.C; ICD-10: C84.4; ICD-9: 202.7] | Phase 2 | [3] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 2 | [2] | ||
| Tumour [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1 | [2] | ||
| Company |
Sirion Therapeutics; cerrx; Sytera Inc
|
|||
| Structure |
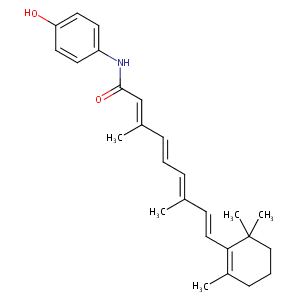 |
Download2D MOL |
||
| Formula |
C26H33NO2
|
|||
| Canonical SMILES |
CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CC(=O)NC2=CC=C(C=C2)O)C)C
|
|||
| InChI |
1S/C26H33NO2/c1-19(11-16-24-21(3)10-7-17-26(24,4)5)8-6-9-20(2)18-25(29)27-22-12-14-23(28)15-13-22/h6,8-9,11-16,18,28H,7,10,17H2,1-5H3,(H,27,29)/b9-6+,16-11+,19-8+,20-18+
|
|||
| InChIKey |
AKJHMTWEGVYYSE-FXILSDISSA-N
|
|||
| CAS Number |
CAS 65646-68-6
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:42588
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Retinoic acid receptor (RAR) | Target Info | Modulator | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00003075) Fenretinide in Treating Patients With Cervical Neoplasia. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | National Cancer Institute Drug Dictionary (drug id 39582). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

