Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08FIX
|
|||
| Former ID |
DIB020629
|
|||
| Drug Name |
OTSSP167
|
|||
| Synonyms |
OTSSP 167; OTSSP-167; AMX10201
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute lymphoblastic leukaemia [ICD-11: 2A85; ICD-10: C85.1, C88.7] | Phase 1 | [1] | |
| Acute myeloid leukaemia [ICD-11: 2A60] | Phase 1 | [1] | ||
| Breast cancer [ICD-11: 2C60-2C65] | Phase 1 | [1] | ||
| Chronic lymphocytic leukaemia [ICD-11: 2A82.0; ICD-10: C83.0, C91.1] | Phase 1 | [1] | ||
| Myelodysplastic syndrome [ICD-11: 2A37; ICD-9: 238.7] | Phase 1 | [1] | ||
| Myeloproliferative neoplasm [ICD-11: 2A20; ICD-10: D47.7] | Phase 1 | [1] | ||
| Structure |
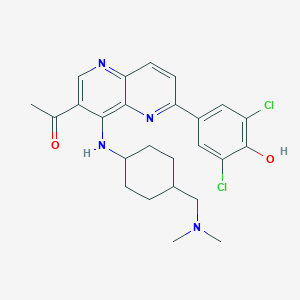 |
Download2D MOL |
||
| Formula |
C25H28Cl2N4O2
|
|||
| Canonical SMILES |
CC(=O)C1=CN=C2C=CC(=NC2=C1NC3CCC(CC3)CN(C)C)C4=CC(=C(C(=C4)Cl)O)Cl
|
|||
| InChI |
1S/C25H28Cl2N4O2/c1-14(32)18-12-28-22-9-8-21(16-10-19(26)25(33)20(27)11-16)30-24(22)23(18)29-17-6-4-15(5-7-17)13-31(2)3/h8-12,15,17,33H,4-7,13H2,1-3H3,(H,28,29)
|
|||
| InChIKey |
DKZYXHCYPUVGAF-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1431697-89-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:95088
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Maternal embryonic leucine zipper kinase (MELK) | Target Info | Inhibitor | [1], [2] |
| Tyrosine-protein kinase MELK (MELK) | Target Info | Inhibitor | [3] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Maternal embryonic leucine zipper kinase is a novel target for proliferation-associated high-risk myeloma.Haematologica. 2018 Feb;103(2):325-335. | |||
| REF 3 | Development of an orally-administrative MELK-targeting inhibitor that suppresses the growth of various types of human cancer. Oncotarget. 2012 Dec;3(12):1629-40. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

