Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08EEU
|
|||
| Former ID |
DIB016258
|
|||
| Drug Name |
Englitazone sodium
|
|||
| Synonyms |
Englitazone sodium < Rec INNM; CP-68722 (racemate); CP-72467-02; CP-72467-2; (-)-5-[2(R)-Benzyl-3,4-dihydro-2H-benzopyran-6-ylmethyl]thiazolidine-2,4-dione sodium salt
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Type-2 diabetes [ICD-11: 5A11; ICD-9: 250] | Phase 1 | [1] | |
| Structure |
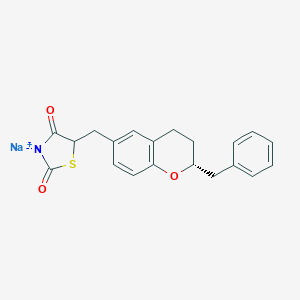 |
Download2D MOL
|
||
| Formula |
C20H18NNaO3S
|
|||
| Canonical SMILES |
C1CC2=C(C=CC(=C2)CC3C(=O)[N-]C(=O)S3)OC1CC4=CC=CC=C4.[Na+]
|
|||
| InChI |
1S/C20H19NO3S.Na/c22-19-18(25-20(23)21-19)12-14-6-9-17-15(10-14)7-8-16(24-17)11-13-4-2-1-3-5-13;/h1-6,9-10,16,18H,7-8,11-12H2,(H,21,22,23);/q;+1/p-1/t16-,18?;/m1./s1
|
|||
| InChIKey |
JQWYNJRCVYGLMO-GPPXSFHXSA-M
|
|||
| CAS Number |
CAS 109229-57-4
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy. Int J Obes Relat Metab Disord. 2003 Feb;27(2):147-61. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

