Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08EBN
|
|||
| Former ID |
DAP001293
|
|||
| Drug Name |
Nitazoxanide
|
|||
| Synonyms |
Alinia; Colufase; Cryptaz; Daxon; Heliton; Nitazoxanid; Nitazoxanida; Nitazoxanidum; Taenitaz; Tizoxanide glucuronide; AZT + Nitazoxanide; Alinia (TN); Annita (TN); Azt+ nitazoxanide; Daxon (TN); Dexidex (TN); Kidonax (TN); Nitax (TN); Nitazox (TN); Nitazoxanida [INN-Spanish]; Nitazoxanide [USAN:INN]; Nitazoxanidum [INN-Latin]; Pacovanton (TN); Paramix (TN); Phavic-1; Zox (TN); Nitazoxanide (USAN/INN); Daxon, Dexidex, Kidonax, Pacovanton, Paramix, Nitax, Zox, Nitazoxanide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Diarrhea [ICD-11: ME05.1; ICD-9: 787.91] | Approved | [1] | |
| Therapeutic Class |
Antiparasitic Agents
|
|||
| Company |
Unimed Pharmaceuticals
|
|||
| Structure |
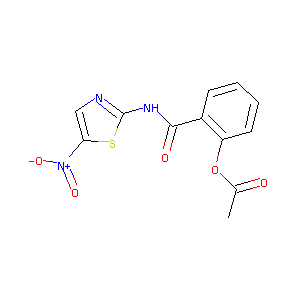 |
Download2D MOL |
||
| Formula |
C12H9N3O5S
|
|||
| Canonical SMILES |
CC(=O)OC1=CC=CC=C1C(=O)NC2=NC=C(S2)[N+](=O)[O-]
|
|||
| InChI |
1S/C12H9N3O5S/c1-7(16)20-9-5-3-2-4-8(9)11(17)14-12-13-6-10(21-12)15(18)19/h2-6H,1H3,(H,13,14,17)
|
|||
| InChIKey |
YQNQNVDNTFHQSW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 55981-09-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
525698, 621648, 3221705, 4644247, 8137752, 8177232, 11439287, 11447486, 12172882, 14800760, 17388853, 17396665, 26719875, 34707303, 46386689, 46507813, 48220047, 48422900, 48425395, 49681590, 50045113, 50955027, 57312593, 58032344, 92251962, 92308454, 92719031, 99431538, 103479736, 104253264, 104337654, 117537931, 118048456, 118315761, 124633706, 124757386, 124889978, 124889979, 124889980, 125164190, 125353161, 125433683, 126628357, 126652269, 126670233, 129833708, 131318910, 134221727, 134338516, 135002935
|
|||
| ChEBI ID |
CHEBI:94807
|
|||
| ADReCS Drug ID | BADD_D01571 | |||
| SuperDrug ATC ID |
P01AX11
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cryptosporidium Pyruvate:ferredoxin oxidoreductase (Crypto CpPNO) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021497. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

