Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08DHN
|
|||
| Former ID |
DIB016939
|
|||
| Drug Name |
VX-787
|
|||
| Synonyms |
Pimodivir
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Influenza A virus infection [ICD-11: 1E30] | Phase 3 | [1] | |
| Company |
Vertex pharmaceuticals
|
|||
| Structure |
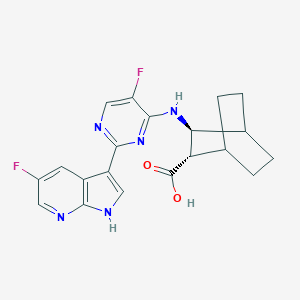 |
Download2D MOL |
||
| Formula |
C20H19F2N5O2
|
|||
| Canonical SMILES |
C1CC2CCC1C(C2NC3=NC(=NC=C3F)C4=CNC5=C4C=C(C=N5)F)C(=O)O
|
|||
| InChI |
1S/C20H19F2N5O2/c21-11-5-12-13(7-24-17(12)23-6-11)18-25-8-14(22)19(27-18)26-16-10-3-1-9(2-4-10)15(16)20(28)29/h5-10,15-16H,1-4H2,(H,23,24)(H,28,29)(H,25,26,27)/t9?,10?,15-,16-/m0/s1
|
|||
| InChIKey |
JGPXDNKSIXAZEQ-SBBZOCNPSA-N
|
|||
| CAS Number |
CAS 1629869-44-8
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Influenza Polymerase basic protein 2 (Influ PB2) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03376321) A Study to Evaluate the Efficacy and Safety of Pimodivir in Combination With the Standard-of-Care Treatment in Adolescent, Adult, and Elderly Hospitalized Participants With Influenza A Infection. U.S. National Institutes of Health. | |||
| REF 2 | Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem. 2014 Aug 14;57(15):6668-78. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

