Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07XOV
|
|||
| Drug Name |
EFT508
|
|||
| Synonyms |
Tomivosertib; eFT508; 1849590-01-7; EFT-508; UNII-U2H19X4WBV; U2H19X4WBV; Tomivosertib [INN]; Tomivosertib [USAN]; SCHEMBL17362622; GTPL10167; eFT-508 (eFT508); EFT 508; MolPort-044-560-418; BCP18993; EX-A2494; ZINC575623807; AKOS030627405; CS-5841; compound 23 [PMID: 29526098]; HY-100022; S8275; 6'-((6-aminopyrimidin-4-yl)amino)-8'-methyl-1'H-spiro[cyclohexane-1,3'-imidazo[1,5-a]pyridine]-1',5'(2'H)-dione; 6-[(6-aminopyrimidin-4-yl)amino]-8-methylspiro[2H-imidazo[1,5-a]pyridine-3,1'-cyclohexane]-1,5-dione
Click to Show/Hide
|
|||
| Indication | Colorectal cancer [ICD-11: 2B91.Z] | Phase 2 | [1] | |
| Hepatocellular carcinoma [ICD-11: 2C12.02; ICD-10: C22.0; ICD-9: 155] | Phase 2 | [1] | ||
| Triple negative breast cancer [ICD-11: 2C60-2C65] | Phase 2 | [1] | ||
| Lymphoma [ICD-11: 2A80-2A86; ICD-9: 202.8, 208.9] | Phase 1/2 | [1] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z] | Phase 1/2 | [1] | ||
| Company |
eFFECTOR Therapeutics San Diego, CA
|
|||
| Structure |
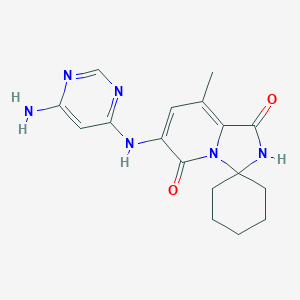 |
Download2D MOL |
||
| Formula |
C17H20N6O2
|
|||
| Canonical SMILES |
CC1=C2C(=O)NC3(N2C(=O)C(=C1)NC4=NC=NC(=C4)N)CCCCC3
|
|||
| InChI |
1S/C17H20N6O2/c1-10-7-11(21-13-8-12(18)19-9-20-13)16(25)23-14(10)15(24)22-17(23)5-3-2-4-6-17/h7-9H,2-6H2,1H3,(H,22,24)(H3,18,19,20,21)
|
|||
| InChIKey |
HKTBYUWLRDZAJK-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1849590-01-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | MAP kinase signal-integrating kinase 2 (MKNK2) | Target Info | Inhibitor | [1] |
| MAPK signal-integrating kinase 1 (MKNK1) | Target Info | Inhibitor | [1] | |
| KEGG Pathway | MAPK signaling pathway | |||
| HIF-1 signaling pathway | ||||
| Insulin signaling pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

