Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07RYI
|
|||
| Drug Name |
Etelcalcetide
|
|||
| Synonyms |
Velcalcetide; Parsabiv; UNII-60ME133FJB; 1262780-97-1; 60ME133FJB; etelcalcetide HCl; Etelcalcetide [USAN:INN]; Etelcalcetide (USAN/INN); Etelcalcetide Hydrochloride(AMG-416)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Secondary hyperparathyroidism [ICD-11: 5A51.1; ICD-10: E21.1] | Approved | [1], [2] | |
| Myelodysplastic syndrome [ICD-11: 2A37; ICD-9: 238.7] | Phase 3 | [3] | ||
| Company |
Amgen/Kai Pharmaceuticals
|
|||
| Structure |
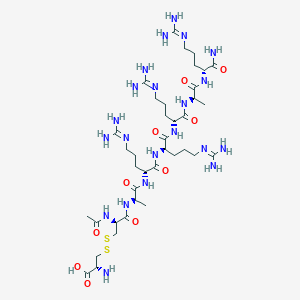 |
Download2D MOL |
||
| Formula |
C38H73N21O10S2
|
|||
| Canonical SMILES |
CC(C(=O)NC(CCCN=C(N)N)C(=O)N)NC(=O)C(CCCN=C(N)N)NC(=O)C(CCCN=C(N)N)NC(=O)C(CCCN=C(N)N)NC(=O)C(C)NC(=O)C(CSSCC(C(=O)O)N)NC(=O)C
|
|||
| InChI |
1S/C38H73N21O10S2/c1-18(28(62)56-22(27(40)61)8-4-12-49-35(41)42)53-30(64)23(9-5-13-50-36(43)44)58-32(66)25(11-7-15-52-38(47)48)59-31(65)24(10-6-14-51-37(45)46)57-29(63)19(2)54-33(67)26(55-20(3)60)17-71-70-16-21(39)34(68)69/h18-19,21-26H,4-17,39H2,1-3H3,(H2,40,61)(H,53,64)(H,54,67)(H,55,60)(H,56,62)(H,57,63)(H,58,66)(H,59,65)(H,68,69)(H4,41,42,49)(H4,43,44,50)(H4,45,46,51)(H4,47,48,52)/t18-,19-,21+,22-,23-,24-,25-,26-/m1/s1
|
|||
| InChIKey |
ANIAZGVDEUQPRI-ZJQCGQFWSA-N
|
|||
| CAS Number |
CAS 1262780-97-1
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:134700
|
|||
| ADReCS Drug ID | BADD_D02514 | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Extracellular calcium-sensing receptor (CASR) | Target Info | Modulator | [4] |
| Pathway Interaction Database | E-cadherin signaling in keratinocytes | |||
| Reactome | G alpha (q) signalling events | |||
| G alpha (i) signalling events | ||||
| Class C/3 (Metabotropic glutamate/pheromone receptors) | ||||
| WikiPathways | GPCRs, Class C Metabotropic glutamate, pheromone | |||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2018 | |||
| REF 2 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||
| REF 3 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031284) | |||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

