Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07QYQ
|
|||
| Drug Name |
Chloroquine
|
|||
| Synonyms |
Amokin; Aralen; Aralen HCl; Arechin; Arechine; Arequin; Arolen; Arthrochin; Artrichin; Avlochlor; Avloclor; Bemaco; Bemaphate; Bemasulph; Benaquin; Bipiquin; CU-01000012392-2; Capquin; Chemochin; Chingamin; Chloraquine; Chlorochin; Chlorochine; Chlorochinum; Chloroin; Chloroquin; Chloroquina; Chloroquine (USP/INN); Chloroquine (VAN); Chloroquine Bis-Phosphoric Acid; Chloroquine FNA (TN); Chloroquine [USAN:INN:BAN]; Chloroquine phosphate; Chloroquinium; Chloroquinum; Chloroquinum [INN-Latin]; Chlorquin; Cidanchin; Clorochina; Clorochina [DCIT]; Cloroquina; Cloroquina [INN-Spanish]; Cocartrit; Dawaquin (TN); Delagil; Dichinalex; Elestol; Gontochin; Gontochin phosphate; Heliopar; Imagon; Ipsen 225; Iroquine; Khingamin; Klorokin; Lapaquin; Malaquin; Malaquin (*Diphosphate*); Malaren; Malarex; Mesylith; Miniquine; Neochin; Nivachine; Nivaquine B; Pfizerquine; Quinachlor; Quinagamin; Quinagamine; Quinercyl; Quingamine; Quinilon; Quinoscan; RP 3377; RP-3377; Resochen; Resochin; Resochin (TN); Resoquina; Resoquine; Reumachlor; Reumaquin; Rivoquine; Ro 01-6014/N2; Ronaquine; Roquine; SN 6718; SN-7618; ST 21; ST 21 (pharmaceutical); Sanoquin; Silbesan;Siragan; Solprina; Sopaquin; Tanakan; Tanakene; Tresochin; Trochin; W 7618;WIN 244
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Phase 3 | [1] | |
| Malaria [ICD-11: 1F40-1F45; ICD-9: 84] | Withdrawn from market | [2] | ||
| Middle East Respiratory Syndrome (MERS) [ICD-11: 1D64] | Investigative | [3] | ||
| Severe acute respiratory syndrome (SARS) [ICD-11: 1D65] | Investigative | [4] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Medreich Sterilab Ltd
|
|||
| Structure |
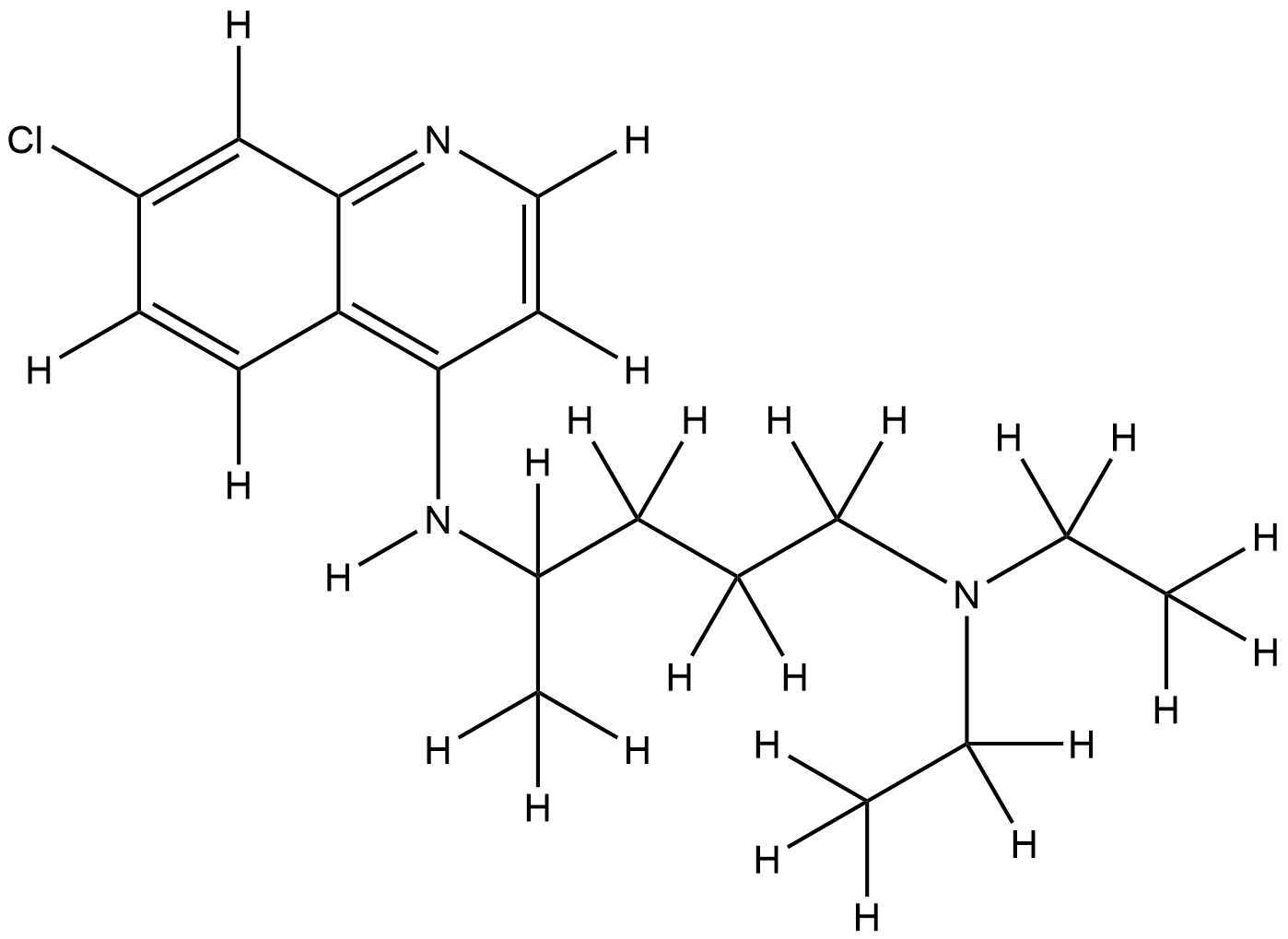 |
Download2D MOL |
||
| Formula |
C18H26ClN3
|
|||
| Canonical SMILES |
CCN(CC)CCCC(C)NC1=C2C=CC(=CC2=NC=C1)Cl
|
|||
| InChI |
1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21)
|
|||
| InChIKey |
WHTVZRBIWZFKQO-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 54-05-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9827, 447935, 596572, 5050990, 7849425, 7978921, 8151759, 10526125, 11335579, 11360818, 11363006, 11365568, 11368130, 11371321, 11373917, 11376292, 11404351, 11405289, 11461790, 11466576, 11467696, 11483910, 11486252, 11487943, 11490157, 11492094, 11493946, 11528736, 14776983, 26675665, 29221876, 46506925, 47217037, 47291356, 47440521, 47440522, 47885638, 48035387, 48110720, 48185227, 48259487, 49635547, 49681217, 49698849, 49846799, 50111008, 53787949, 53788124, 56310729, 57321414
|
|||
| ChEBI ID |
CHEBI:3638
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN glycosylation of host receptor (GHR) | Target Info | Inhibitor | [5], [6] |
| HUMAN pH-dependent viral fusion/replication (pH-DVF/R) | Target Info | Inhibitor | [5], [6] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04360759) Chloroquine Outpatient Treatment Evaluation for HIV-Covid-19. U.S. National Institutes of Health. | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47. | |||
| REF 4 | Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020 Mar;19(3):149-150. | |||
| REF 5 | Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Mar;30(3):269-271. | |||
| REF 6 | Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol. 2020 Apr;15(4):247-249. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

