Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07NVU
|
|||
| Former ID |
DCL000165
|
|||
| Drug Name |
Midostaurin
|
|||
| Synonyms |
PKC412; 120685-11-2; Cgp 41251; 4'-N-Benzoylstaurosporine; CGP-41251; Benzoylstaurosporine; PKC-412; RYDAPT; PKC 412; UNII-ID912S5VON; N-Benzoylstaurosporine; ID912S5VON; CHEMBL608533; CHEBI:63452; Cgp 41 251; N-[(5S,6R,7R,9R)-6-methoxy-5-methyl-14-oxo-6,7,8,9,15,16-hexahydro-5H,14H-5,9-epoxy-4b,9a,15-triazadibenzo[b,h]cyclonona[1,2,3,4-jkl]cyclopenta[e]-as-indacen-7-yl]-N-methylbenzamide; PKC-412(Midostaurin); Midostaurin (PKC412); Midostaurin (USAN/INN); Midostaurin [USAN:INN]; CGP 41231; Rydapt (TN); CPG 41251
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute myeloid leukaemia [ICD-11: 2A60] | Approved | [1] | |
| Systemic mastocytosis [ICD-11: 2A21.0; ICD-9: 202.6, 757.33] | Approved | [2] | ||
| Chronic myelomonocytic leukaemia [ICD-11: 2A40; ICD-10: C93.1] | Phase 2 | [3], [4] | ||
| Colorectal cancer [ICD-11: 2B91.Z] | Phase 1 | [3], [4] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Novartis
|
|||
| Structure |
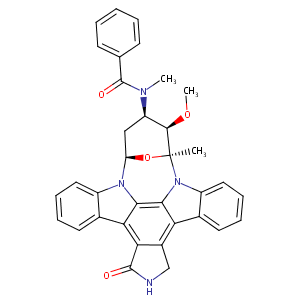 |
Download2D MOL
|
||
| Formula |
C35H30N4O4
|
|||
| Canonical SMILES |
CC12C(C(CC(O1)N3C4=CC=CC=C4C5=C6C(=C7C8=CC=CC=C8N2C7=C53)CNC6=O)N(C)C(=O)C9=CC=CC=C9)OC
|
|||
| InChI |
1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1
|
|||
| InChIKey |
BMGQWWVMWDBQGC-IIFHNQTCSA-N
|
|||
| CAS Number |
CAS 120685-11-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14788708, 14935507, 22395186, 44927646, 47206756, 53786846, 57373453, 79311635, 99302777, 103734272, 123105168, 124659175, 124950161, 134348392, 135061643, 135610396, 137241200, 143298037, 172918683, 174006478, 177748492, 178102329, 179149698, 184812273, 198965438, 210274665, 210280297, 223656331, 233822082, 241376205, 248897758, 249617730, 252156932
|
|||
| ChEBI ID |
CHEBI:63452
|
|||
| ADReCS Drug ID | BADD_D02453 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2018 | |||
| REF 2 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5702). | |||
| REF 4 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | |||
| REF 5 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||
| REF 6 | CBL exon 8/9 mutants activate the FLT3 pathway and cluster in core binding factor/11q deletion acute myeloid leukemia/myelodysplastic syndrome subt... Clin Cancer Res. 2009 Apr 1;15(7):2238-47. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

