Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07NOI
|
|||
| Former ID |
DCL000356
|
|||
| Drug Name |
BAY-57-9352
|
|||
| Synonyms |
Telatinib; Bay 57-9352
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Gastric adenocarcinoma [ICD-11: 2B72; ICD-9: 151] | Phase 2 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 2 | [2] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Bayer AG
|
|||
| Structure |
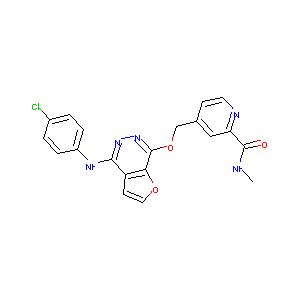 |
Download2D MOL |
||
| Formula |
C20H16ClN5O3
|
|||
| Canonical SMILES |
CNC(=O)C1=NC=CC(=C1)COC2=NN=C(C3=C2OC=C3)NC4=CC=C(C=C4)Cl
|
|||
| InChI |
1S/C20H16ClN5O3/c1-22-19(27)16-10-12(6-8-23-16)11-29-20-17-15(7-9-28-17)18(25-26-20)24-14-4-2-13(21)3-5-14/h2-10H,11H2,1H3,(H,22,27)(H,24,25)
|
|||
| InChIKey |
QFCXANHHBCGMAS-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 332012-40-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14831018, 24115824, 44844835, 75059184, 123051133, 124360774, 124757885, 124955475, 125164687, 126661316, 126731532, 131477698, 135260928, 135367560, 136348776, 136349488, 136367619, 136367765, 137156647, 143010267, 152048944, 160680893, 162011760, 162038009, 162202687, 163348562, 163908069, 164042063, 164765235, 170502613, 172919643, 174528808, 198939231, 223396209, 223705027, 227479450, 242060040, 245318899, 251971207, 252110147, 252216386
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021605) | |||
| REF 3 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

