Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07KYT
|
|||
| Former ID |
DNCL002109
|
|||
| Drug Name |
Quizartinib
|
|||
| Synonyms |
AC220
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute myeloid leukaemia [ICD-11: 2A60; ICD-9: 205] | Approved | [1] | |
| leukaemia [ICD-11: 2A60-2B33; ICD-9: 208.9] | Phase 3 | [2] | ||
| Company |
Daiichi Sankyo
|
|||
| Structure |
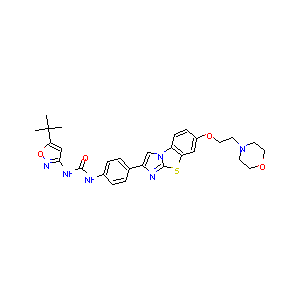 |
Download2D MOL |
||
| Formula |
C29H32N6O4S
|
|||
| Canonical SMILES |
CC(C)(C)C1=CC(=NO1)NC(=O)NC2=CC=C(C=C2)C3=CN4C5=C(C=C(C=C5)OCCN6CCOCC6)SC4=N3
|
|||
| InChI |
1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36)
|
|||
| InChIKey |
CVWXJKQAOSCOAB-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 950769-58-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
51081888, 57459864, 99437205, 103699058, 104155051, 124757317, 124950695, 125164121, 125329937, 135264521, 135626680, 135685330, 135685331, 135685350, 135686086, 135686087, 135686104, 135686105, 135727410, 135727821, 136350019, 136367457, 136368137, 136920258, 137262639, 137275856, 141168277, 144115531, 152030976, 152258408, 160647245, 162011412, 162037647, 162189542, 163775377, 164777892, 174561046, 177749071, 178102286, 180371638, 188899552, 196409687, 198974101, 223705253, 224646788, 226453032, 243160980, 249814511, 249818724, 249869565
|
|||
| ChEBI ID |
CHEBI:90217
|
|||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Fms-like tyrosine kinase 3 (FLT-3) | Target Info | Inhibitor | [1] |
| KEGG Pathway | Cytokine-cytokine receptor interaction | |||
| Hematopoietic cell lineage | ||||
| Pathways in cancer | ||||
| Transcriptional misregulation in cancer | ||||
| Acute myeloid leukemia | ||||
| Central carbon metabolism in cancer | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 216993 | |||
| REF 2 | ClinicalTrials.gov (NCT02039726) An Open-label Study of Quizartinib Monotherapy vs. Salvage Chemotherapy in Acute Myeloid Leukemia (AML) Subjects. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

