Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07JVU
|
|||
| Drug Name |
Ciclosporin
|
|||
| Synonyms |
Cyclosporine A; CSA; CsA; Antibiotic S 7481F1; BMT-ABA-SAR-MLE-VAL-MLE-ALA-ALA-MLE-MLE-MVA; BMT-ABA-SAR-MLE-VAL-MLE-ALA-DAL-MLE-MLE-MVA; C 3662; CB-01-09 MMX; CYCLOSPORIN A (SEE ALSO TRANSGENIC MODEL EVALUATION (CYCLOSPORIN A)); Cicloral (TN); Ciclosporin; Ciclosporin (JP15); Cipol N; Cipol-N; Consupren; Consupren S; CsA & IFN-alpha; Cyclokat; Cyclophorine; Cyclosporin; Cyclosporin A; Cyclosporin A & IFN-alpha; Cyclosporin A Implant; Cyclosporin A, Tolypocladium inflatum; Cyclosporine (USP); Cyclosporine A; Cyclosporine [USAN]; DE-076; Equoral; From Tolypocladium inflatum (Trichoderma polysporin); GNF-Pf-2808; Gengraf; Gengraf (TN); Helv Chim Acta 60: 1568 (1977); Mitogard; Modusik-A; Neoplanta; Neoral; Neoral (TN); NeuroSTAT; Nova-22007; OL 27-400; OL-27400; OLO-400; Papilock; Pulminiq; RamihyphinA; Restasis; Restasis (TN); S-Neoral; SDZ-OXL 400; ST-603; Sandimmun; Sandimmun Neoral; Sandimmune; Sandimmune (TN); Sandimmune, Gengraf, Restasis, Atopica, Sangcya, Cyclosporine; Sang-2000; Sang-35; SangCyA; Sigmasporin; Sigmasporin Microoral; TRANSGENIC MODEL EVALUATION (CYCLOSPORIN A); Vekacia; Zyclorin
Click to Show/Hide
|
|||
| Drug Type |
Protein/peptide drug
|
|||
| Indication | Graft-versus-host disease [ICD-11: 4B24] | Approved | [1] | |
| Middle East Respiratory Syndrome (MERS) [ICD-11: 1D64] | Investigative | [2], [3] | ||
| Severe acute respiratory syndrome (SARS) [ICD-11: 1D65] | Investigative | [2], [3] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Sandoz
|
|||
| Structure |
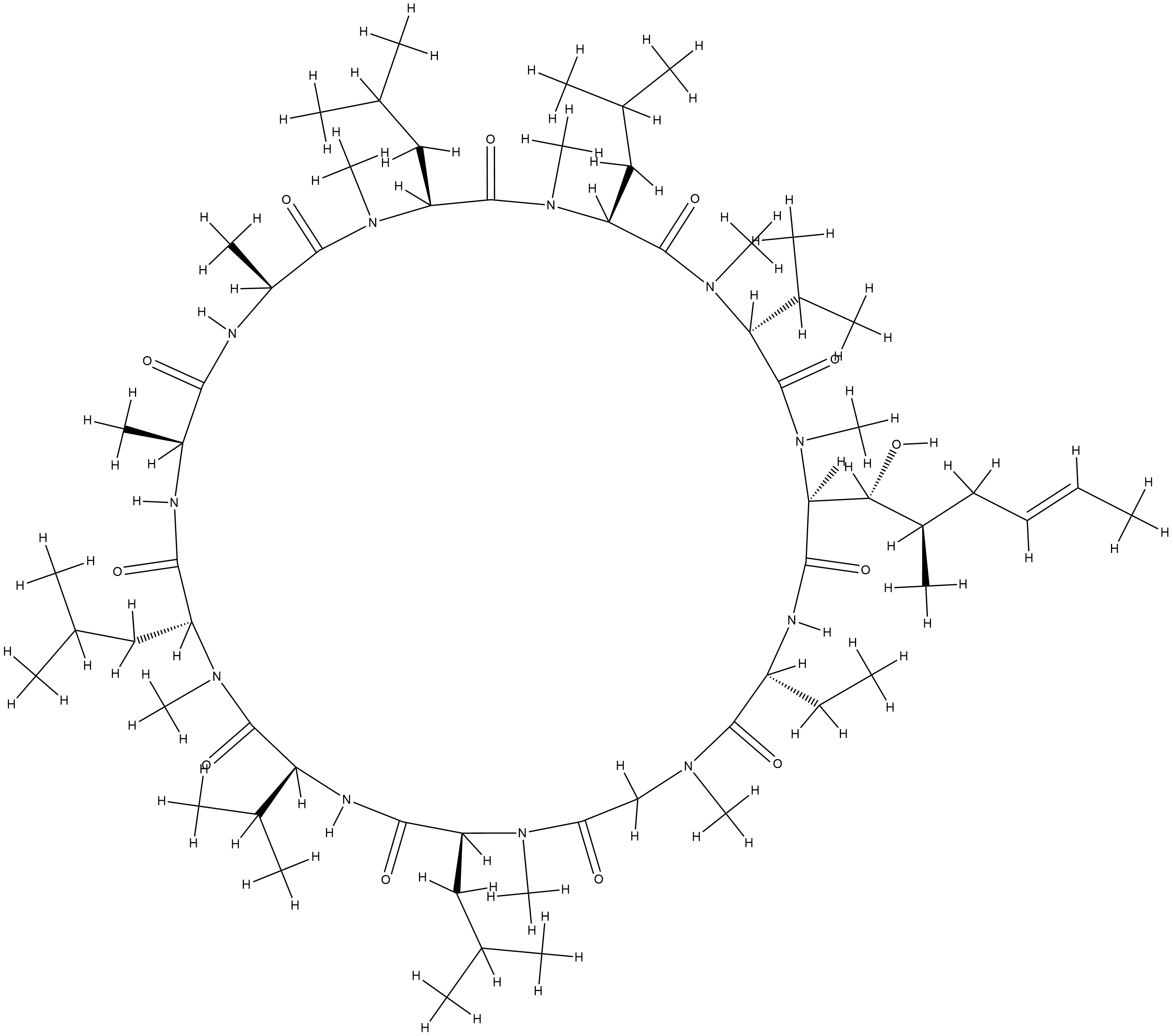 |
Download2D MOL
|
||
| Canonical SMILES |
CCC1C(=O)N(CC(=O)N(C(C(=O)NC(C(=O)N(C(C(=O)NC(C(=O)NC(C(=O)N(C(C(=O)N(C(C(=O)N(C(C(=O)N(C(C(=O)N1)C(C(C)CC=CC)O)C)C(C)C)C)CC(C)C)C)CC(C)C)C)C)C)CC(C)C)C)C(C)C)CC(C)C)C)C
|
|||
| CAS Number |
CAS 59865-13-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
127280714, 127280715, 127280716, 127280717, 127280718, 127280719, 127280720, 127280721, 127280722, 127280723, 127280724, 127280725, 127280726, 127280727, 127280728, 127280729, 127280730, 127280731, 127280732, 127300898, 127300899, 127300900, 127300901, 127300902, 127300903, 127300904, 127300905, 127300906, 127300907, 127300908, 127300909, 127300910, 127300911, 127300912, 127300913, 127300914, 137018976, 226407640
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN cyclophilin D (CYP3) | Target Info | Inhibitor | [2], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020 Mar;19(3):149-150. | |||
| REF 3 | Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

