Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07GXR
|
|||
| Former ID |
DNCL003827
|
|||
| Drug Name |
Ubrogepant
|
|||
| Synonyms |
1374248-77-7; UNII-AD0O8X2QJR; MK-1602; AD0O8X2QJR; Ubrogepant [USAN:INN]; Ubrogepant (USAN/INN); SCHEMBL3698428; CHEMBL2364638; GTPL10176; DTXSID00160178; DDOOFTLHJSMHLN-ZQHRPCGSSA-N; MK1602; ZINC95598454; SB19741; HY-12366; CS-0011109; D10673; (3S)-N-[(3S,5S,6R)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-3-yl]-2-oxospiro[1H-pyrrolo[2,3-b]pyridine-3,6'-5,7-dihydrocyclopenta[b]pyridine]-3'-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Migraine [ICD-11: 8A80; ICD-10: G43, G43.9; ICD-9: 346] | Approved | [1] | |
| Company |
Merck
|
|||
| Structure |
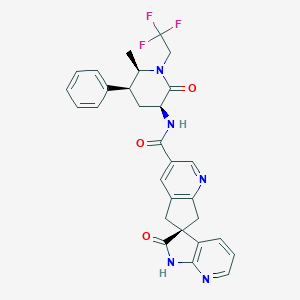 |
Download2D MOL |
||
| Formula |
C29H26F3N5O3
|
|||
| Canonical SMILES |
CC1C(CC(C(=O)N1CC(F)(F)F)NC(=O)C2=CC3=C(CC4(C3)C5=C(NC4=O)N=CC=C5)N=C2)C6=CC=CC=C6
|
|||
| InChI |
1S/C29H26F3N5O3/c1-16-20(17-6-3-2-4-7-17)11-22(26(39)37(16)15-29(30,31)32)35-25(38)19-10-18-12-28(13-23(18)34-14-19)21-8-5-9-33-24(21)36-27(28)40/h2-10,14,16,20,22H,11-13,15H2,1H3,(H,35,38)(H,33,36,40)/t16-,20-,22+,28+/m1/s1
|
|||
| InChIKey |
DDOOFTLHJSMHLN-ZQHRPCGSSA-N
|
|||
| CAS Number |
CAS 1374248-77-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Calcitonin gene-related peptide receptor (CGRPR) | Target Info | Antagonist | [1] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Vascular smooth muscle contraction | ||||
| Reactome | G alpha (s) signalling events | |||
| Calcitonin-like ligand receptors | ||||
| WikiPathways | GPCRs, Class B Secretin-like | |||
| Endothelin Pathways | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

