Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07FKQ
|
|||
| Former ID |
DNCL003649
|
|||
| Drug Name |
Siponimod
|
|||
| Synonyms |
BAF312
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Multiple sclerosis [ICD-11: 8A40] | Approved | [1] | |
| Hepatocellular carcinoma [ICD-11: 2C12.02; ICD-10: C22.0] | Phase 3 | [2] | ||
| Rheumatoid arthritis [ICD-11: FA20] | Phase 2 | [3], [4] | ||
| Company |
Novartis
|
|||
| Structure |
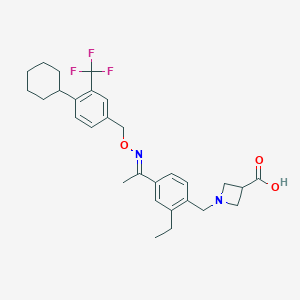 |
Download2D MOL |
||
| Formula |
C29H35F3N2O3
|
|||
| Canonical SMILES |
CCC1=C(C=CC(=C1)C(=NOCC2=CC(=C(C=C2)C3CCCCC3)C(F)(F)F)C)CN4CC(C4)C(=O)O
|
|||
| InChI |
1S/C29H35F3N2O3/c1-3-21-14-23(10-11-24(21)15-34-16-25(17-34)28(35)36)19(2)33-37-18-20-9-12-26(22-7-5-4-6-8-22)27(13-20)29(30,31)32/h9-14,22,25H,3-8,15-18H2,1-2H3,(H,35,36)/b33-19+
|
|||
| InChIKey |
KIHYPELVXPAIDH-HNSNBQBZSA-N
|
|||
| CAS Number |
CAS 1230487-00-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ADReCS Drug ID | BADD_D02511 | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Sphingosine-1 phosphate receptor (S1PR) | Target Info | Modulator | [1] |
| Sphingosine-1-phosphate receptor 1 (S1PR1) | Target Info | Modulator | [1] | |
| KEGG Pathway | FoxO signaling pathway | |||
| Sphingolipid signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| TGF_beta_Receptor Signaling Pathway | ||||
| IL2 Signaling Pathway | ||||
| Pathway Interaction Database | Fc-epsilon receptor I signaling in mast cells | |||
| S1P3 pathway | ||||
| S1P1 pathway | ||||
| Sphingosine 1-phosphate (S1P) pathway | ||||
| PDGFR-beta signaling pathway | ||||
| Reactome | G alpha (i) signalling events | |||
| Lysosphingolipid and LPA receptors | ||||
| WikiPathways | Signal Transduction of S1P Receptor | |||
| Small Ligand GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | |||
| REF 2 | ClinicalTrials.gov (NCT01665144) Exploring the Efficacy and Safety of Siponimod in Patients With Secondary Progressive Multiple Sclerosis (EXPAND). U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

