Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06YQR
|
|||
| Former ID |
DNC010720
|
|||
| Drug Name |
(2R,3S,4S,5R)-2-propylpiperidine-3,4,5-triol
|
|||
| Synonyms |
CHEMBL1089185; (2R,3S,4S,5R)-2-propylpiperidine-3,4,5-triol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
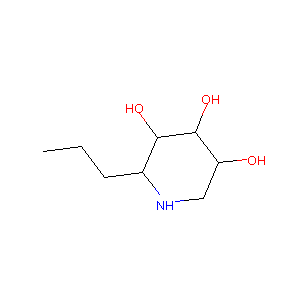 |
Download2D MOL |
||
| Formula |
C8H17NO3
|
|||
| Canonical SMILES |
CCCC1C(C(C(CN1)O)O)O
|
|||
| InChI |
1S/C8H17NO3/c1-2-3-5-7(11)8(12)6(10)4-9-5/h5-12H,2-4H2,1H3/t5-,6-,7+,8+/m1/s1
|
|||
| InChIKey |
JFOGNLDWQBZRQJ-NGJRWZKOSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glucosylceramidase (GBA) | Target Info | Inhibitor | [1] |
| KEGG Pathway | Other glycan degradation | |||
| Sphingolipid metabolism | ||||
| Metabolic pathways | ||||
| Lysosome | ||||
| Pathwhiz Pathway | Sphingolipid Metabolism | |||
| Reactome | Glycosphingolipid metabolism | |||
| WikiPathways | Sphingolipid metabolism | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Synthesis of new beta-1-C-alkylated imino-L-iditols: A comparative study of their activity as beta-glucocerebrosidase inhibitors. Bioorg Med Chem. 2010 Apr 1;18(7):2645-50. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

