Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06XGC
|
|||
| Former ID |
DIB002553
|
|||
| Drug Name |
BMS-986202
|
|||
| Synonyms |
AM-095; AM-966; LPA1 antagonists (fibrotic disease), Amira; Lysophosphatidic acid antagonists (fibrotic disease), Amira
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Idiopathic pulmonary fibrosis [ICD-11: CB03.4; ICD-10: J84.1; ICD-9: 516.3] | Preclinical | [1], [2] | |
| Company |
Bristol-myers squibb; Amira Pharmaceuticals Inc
|
|||
| Structure |
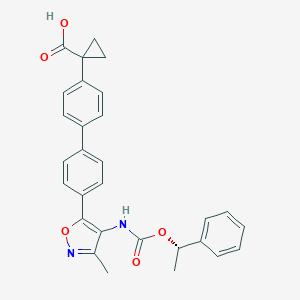 |
Download2D MOL |
||
| Formula |
C29H26N2O5
|
|||
| Canonical SMILES |
CC1=NOC(=C1NC(=O)OC(C)C2=CC=CC=C2)C3=CC=C(C=C3)C4=CC=C(C=C4)C5(CC5)C(=O)O
|
|||
| InChI |
1S/C29H26N2O5/c1-18-25(30-28(34)35-19(2)20-6-4-3-5-7-20)26(36-31-18)23-10-8-21(9-11-23)22-12-14-24(15-13-22)29(16-17-29)27(32)33/h3-15,19H,16-17H2,1-2H3,(H,30,34)(H,32,33)/t19-/m0/s1
|
|||
| InChIKey |
GQBRZBHEPUQRPL-IBGZPJMESA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Lysophosphatidic acid receptor 1 (LPAR1) | Target Info | Antagonist | [3] |
| KEGG Pathway | Rap1 signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| PI3K-Akt signaling pathway | ||||
| Gap junction | ||||
| Pathways in cancer | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | |||
| Pathway Interaction Database | LPA receptor mediated events | |||
| Reactome | G alpha (q) signalling events | |||
| G alpha (i) signalling events | ||||
| Lysosphingolipid and LPA receptors | ||||
| WikiPathways | Myometrial Relaxation and Contraction Pathways | |||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Small Ligand GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6988). | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800030351) | |||
| REF 3 | Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J Pharmacol Exp Ther. 2011 Mar;336(3):693-700. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

