Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06VRI
|
|||
| Drug Name |
Leflunomide
|
|||
| Synonyms |
leflunomide; 75706-12-6; Arava; lefunamide; Leflunomidum; Leflunomida; HWA 486; Leflunomidum [INN-Latin]; HWA-486; Leflunomida [INN-Spanish]; SU 101 (pharmaceutical); SU101; Arava (TN); UNII-G162GK9U4W; S; Leflunomide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Multiple sclerosis [ICD-11: 8A40] | Approved | [1] | |
| Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Phase 1 | [2] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
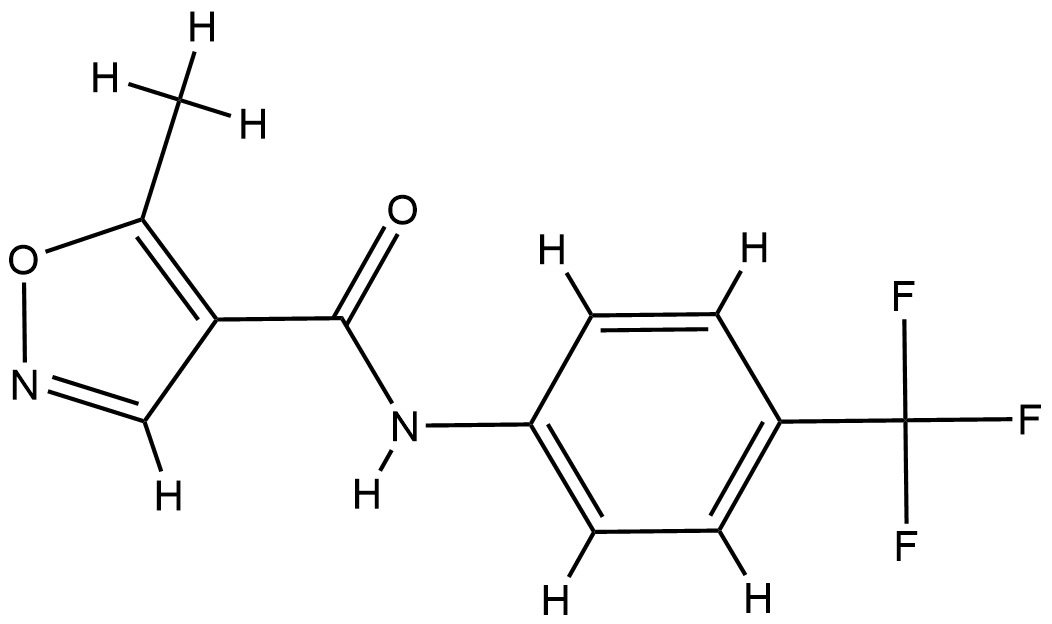 |
Download2D MOL |
||
| Formula |
C12H9F3N2O2
|
|||
| Canonical SMILES |
CC1=C(C=NO1)C(=O)NC2=CC=C(C=C2)C(F)(F)F
|
|||
| InChI |
1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18)
|
|||
| InChIKey |
VHOGYURTWQBHIL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 75706-12-6
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:6402
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN dihydroorotate dehydrogenase (DHODH) | Target Info | Inhibitor | [3], [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 077090. | |||
| REF 2 | ClinicalTrials.gov (NCT04361214) Leflunomide in Mild COVID-19 Patients. U.S. National Institutes of Health. | |||
| REF 3 | On dihydroorotate dehydrogenases and their inhibitors and uses. J Med Chem. 2013 Apr 25;56(8):3148-67. doi: 10.1021/jm301848w. | |||
| REF 4 | IMU-838 Targeting DHODH | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

