Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06TFI
|
|||
| Former ID |
DNCL002630
|
|||
| Drug Name |
LY2157299
|
|||
| Synonyms |
Galunisertib; 700874-72-2; LY2157299; LY 2157299; LY-2157299; UNII-3OKH1W5LZE; 4-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)quinoline-6-carboxamide; 3OKH1W5LZE; Galunisertib (LY2157299); AK-79916; 4-[5,6-Dihydro-2-(6-methyl-2-pyridinyl)-4H-pyrrolo[1,2-b]pyrazol-3-yl]-6-quinolinecarboxamide; 4-(2-(6-Methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo-[1,2-b]pyrazol-3-yl)quinoline-6-carboxamide; 4-[2-(6-methylpyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline-6-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Arteriosclerosis [ICD-11: BD40; ICD-9: 440] | Phase 2/3 | [1], [2] | |
| Non-small-cell lung cancer [ICD-11: 2C25.Y; ICD-9: 162] | Phase 2/3 | [3] | ||
| Hepatocellular carcinoma [ICD-11: 2C12.02; ICD-10: C22.0; ICD-9: 155] | Phase 2 | [3], [4] | ||
| Glioma [ICD-11: 2A00.0; ICD-9: 191] | Phase 1/2 | [4] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1/2 | [3] | ||
| Pancreatic cancer [ICD-11: 2C10; ICD-9: 185] | Phase 1 | [3] | ||
| Company |
Eli Lilly
|
|||
| Structure |
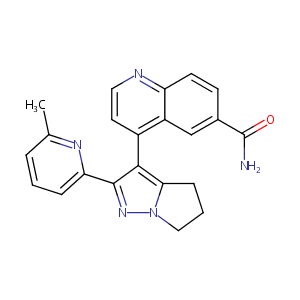 |
Download2D MOL |
||
| Formula |
C22H19N5O
|
|||
| Canonical SMILES |
CC1=NC(=CC=C1)C2=NN3CCCC3=C2C4=C5C=C(C=CC5=NC=C4)C(=O)N
|
|||
| InChI |
1S/C22H19N5O/c1-13-4-2-5-18(25-13)21-20(19-6-3-11-27(19)26-21)15-9-10-24-17-8-7-14(22(23)28)12-16(15)17/h2,4-5,7-10,12H,3,6,11H2,1H3,(H2,23,28)
|
|||
| InChIKey |
IVRXNBXKWIJUQB-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 700874-72-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
15077613, 22501521, 39150367, 74882604, 123051136, 124360776, 125164689, 126659528, 131324157, 135697765, 135727455, 136349487, 136367618, 139858822, 143499314, 152234786, 152258470, 160647305, 162011359, 162038034, 162108683, 162202753, 162640351, 163913496, 164044969, 164141653, 164223108, 172232530, 172919195, 174530317, 185968804, 198946153, 208265509, 215774683, 223366128, 223400636, 223404315, 223434994, 223496588, 223556705, 237933695, 242041337, 243051032, 249814489, 251971217, 252125728, 252160190, 252215728, 252451680
|
|||
| ChEBI ID |
CHEBI:137064
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7797). | |||
| REF 2 | ClinicalTrials.gov (NCT02008318) A Study of LY2157299 in Participants With Myelodysplastic Syndromes. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 5 | Cardiac Safety of TGF-beta Receptor I Kinase Inhibitor LY2157299 Monohydrate in Cancer Patients in a First-in-Human Dose Study. Cardiovasc Toxicol. 2015 Oct;15(4):309-23. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

