Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06MMN
|
|||
| Former ID |
DIB019516
|
|||
| Drug Name |
PMID7966163C4e
|
|||
| Synonyms |
GTPL3090; BDBM50038138
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1], [2] | |
| Structure |
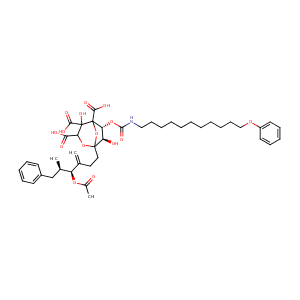 |
Download2D MOL
|
||
| Formula |
C43H57NO15
|
|||
| Canonical SMILES |
CC(CC1=CC=CC=C1)C(C(=C)CCC23C(C(C(O2)(C(C(O3)C(=O)O)(C(=O)O)O)C(=O)O)OC(=O)NCCCCCCCCCCCOC4=CC=CC=C4)O)OC(=O)C
|
|||
| InChI |
1S/C43H57NO15/c1-28(33(56-30(3)45)29(2)27-31-19-13-11-14-20-31)23-24-41-34(46)35(43(59-41,39(51)52)42(54,38(49)50)36(58-41)37(47)48)57-40(53)44-25-17-9-7-5-4-6-8-10-18-26-55-32-21-15-12-16-22-32/h11-16,19-22,29,33-36,46,54H,1,4-10,17-18,23-27H2,2-3H3,(H,44,53)(H,47,48)(H,49,50)(H,51,52)/t29-,33-,34-,35-,36?,41?,42?,43?/m1/s1
|
|||
| InChIKey |
QOLHWXNSCZGWHK-BWBORTOCSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Squalene synthetase (FDFT1) | Target Info | Inhibitor | [1] |
| BioCyc | Cholesterol biosynthesis II (via 24,25-dihydrolanosterol) | |||
| Cholesterol biosynthesis III (via desmosterol) | ||||
| Cholesterol biosynthesis I | ||||
| Superpathway of cholesterol biosynthesis | ||||
| Epoxysqualene biosynthesis | ||||
| KEGG Pathway | Steroid biosynthesis | |||
| Metabolic pathways | ||||
| Biosynthesis of antibiotics | ||||
| Panther Pathway | Cholesterol biosynthesis | |||
| Pathwhiz Pathway | Steroid Biosynthesis | |||
| Reactome | Cholesterol biosynthesis | |||
| PPARA activates gene expression | ||||
| Activation of gene expression by SREBF (SREBP) | ||||
| WikiPathways | Statin Pathway | |||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | ||||
| Activation of Gene Expression by SREBP (SREBF) | ||||
| SREBP signalling | ||||
| Cholesterol Biosynthesis | ||||
| Cholesterol biosynthesis | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Structure-activity relationships of C1 and C6 side chains of zaragozic acid A derivatives. J Med Chem. 1994 Nov 11;37(23):4031-51. | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3090). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

