Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06JPB
|
|||
| Former ID |
DAP000291
|
|||
| Drug Name |
Ergocalciferol
|
|||
| Synonyms |
Calciferol; Calciferolum; Condacaps; Condocaps; Condol; Crtron; Crystallina; Daral; Davitin; Decaps; Deltalin; Deratol; Detalup; Diactol; Drisdol; Ercalciol; Ergocalciferolo; Ergocalciferols; Ergocalciferolum; Ergorone; Ertron; Fortodyl; Geltabs; Haliver; Hyperkil; Infron; Metadee; Mulsiferol; Mykostin; Ostelin; Radiostol; Radstein; Radsterin; Rodinec; Sterogyl; Vigantol; Viostdrol; Viosterol; CALCIFEROL IN A GELATIN MATRIX; Component of Geltabs Vitamin D; Davitamon D; Divit urto; Ergocalciferolo [DCIT]; Ergosterol activated; Ergosterol irradiated; Geltabs Vitamin D; Irradiated ergosterol; Oleovitamin D; Rodine C; Synthetic Vitamin D; Viosterol in Oil; Calciferon 2; Mina D2; Oleovitamin D2; VITAMIN D2; VITAMIN_D2; Vitamina D2; Buco-D; Calciferol (TN); Calciferol (vitamin D2); D-Arthin; D-Tracetten; De-rat concentrate; Dee-Osterol; Dee-Ron; Dee-Ronal; Dee-Roual; Deltalin (TN); Drisdol (TN); Ergocalciferol (D2); Ergocalciferol: Vitamin D; Ergocalciferolum [INN-Latin]; Ergosterol, irradiated; Hi-Deratol; Novovitamin-D; Oleovitamin D, Synthetic; Shock-ferol; Shock-ferol sterogyl; Sorex C.R; Uvesterol-D; Vio-D; Vitamin d-2; Vitamin-D2; Vitavel-D; Ergocalciferol (JP15/USP); Ergocalciferol [INN:BAN:JAN]; Sorex C.R.; VITAMIN D2 WATER DISPERSABLE U.S.P.; CALCIFEROL, U.S.P.; Irradiated ergosta-5,7,22-trien-3-beta-ol; Irradiated ergosta-5,7,22-trien-3.beta.-ol; (+)-Vitamin D2; (3-beta,5Z,7E,22E)-9,10-Secoergosta-5,7,10,(19),22-tetraen-3-ol; (3S,5Z,7E,14xi,17alpha,22E)-9,10-secoergosta-5,7,10,22-tetraen-3-ol; (3S,5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraen-3-ol; (3S,5Z,7E,22E)-9,10-secoergosta-5,7,10,22-tetraen-3-ol; (3beta,5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraen-3-ol; (5Z,7E,22E)-(3S)-9,10-seco-5,7,10(19),22-ergostatetraen-3-ol; (5Z,7E,22E)-(3S)-9,10-secoergosta-5,7,10(19),22-tetraen-3-ol; 7E677DC1-E1C4-4FC5-8F4A-BCE1857F7E87; 9,10,Secoergosta-5,7,10(19),22-tetraen 3.beta.-ol;9,10-Seco(5Z,7E,22E)-5,7,10(19),22-ergostatetraen-3-ol; 9,10-Secoergosta-5,7,10(19),22-tetraen-3-beta-ol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypoparathyroidism [ICD-11: 5A50; ICD-9: 252.1] | Approved | [1] | |
| Therapeutic Class |
Vitamins
|
|||
| Company |
Eli Lilly
|
|||
| Structure |
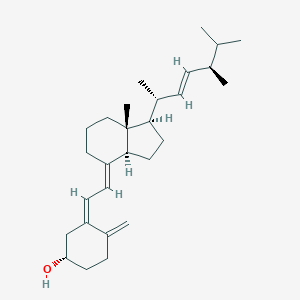 |
Download2D MOL |
||
| Formula |
C28H44O
|
|||
| Canonical SMILES |
CC(C)C(C)C=CC(C)C1CCC2C1(CCCC2=CC=C3CC(CCC3=C)O)C
|
|||
| InChI |
1S/C28H44O/c1-19(2)20(3)9-10-22(5)26-15-16-27-23(8-7-17-28(26,27)6)12-13-24-18-25(29)14-11-21(24)4/h9-10,12-13,19-20,22,25-27,29H,4,7-8,11,14-18H2,1-3,5-6H3/b10-9+,23-12+,24-13-/t20-,22+,25-,26+,27-,28+/m0/s1
|
|||
| InChIKey |
MECHNRXZTMCUDQ-RKHKHRCZSA-N
|
|||
| CAS Number |
CAS 50-14-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:28934
|
|||
| ADReCS Drug ID | BADD_D00794 | |||
| SuperDrug ATC ID |
A11CC01
|
|||
| SuperDrug CAS ID |
cas=000050146
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Vitamin D3 receptor (VDR) | Target Info | Antagonist | [2], [3] |
| KEGG Pathway | Endocrine and other factor-regulated calcium reabsorption | |||
| Mineral absorption | ||||
| Tuberculosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Panther Pathway | Vitamin D metabolism and pathway | |||
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | |||
| Direct p53 effectors | ||||
| RXR and RAR heterodimerization with other nuclear receptor | ||||
| Retinoic acid receptors-mediated signaling | ||||
| Validated transcriptional targets of deltaNp63 isoforms | ||||
| Validated transcriptional targets of TAp63 isoforms | ||||
| Reactome | Nuclear Receptor transcription pathway | |||
| WikiPathways | Ovarian Infertility Genes | |||
| Nuclear Receptors in Lipid Metabolism and Toxicity | ||||
| Nuclear Receptors Meta-Pathway | ||||
| Vitamin D Receptor Pathway | ||||
| Drug Induction of Bile Acid Pathway | ||||
| Nuclear Receptors | ||||
| Vitamin D Metabolism | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040833. | |||
| REF 2 | Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008 Sep;3(5):1535-41. | |||
| REF 3 | Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1689S-96S. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

