Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06DCK
|
|||
| Former ID |
DNC006483
|
|||
| Drug Name |
L-746530
|
|||
| Synonyms |
L-746530; CHEMBL207057; BDBM50182300
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
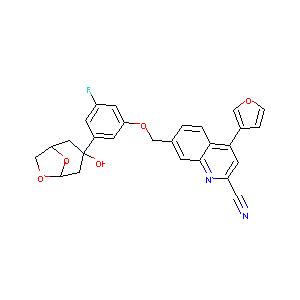 |
Download2D MOL |
||
| Formula |
C27H21FN2O5
|
|||
| Canonical SMILES |
C1C2COC(O2)CC1(C3=CC(=CC(=C3)F)OCC4=CC5=NC(=CC(=C5C=C4)C6=COC=C6)C#N)O
|
|||
| InChI |
1S/C27H21FN2O5/c28-19-6-18(27(31)10-22-15-34-26(11-27)35-22)7-21(8-19)33-13-16-1-2-23-24(17-3-4-32-14-17)9-20(12-29)30-25(23)5-16/h1-9,14,22,26,31H,10-11,13,15H2/t22-,26+,27-/m0/s1
|
|||
| InChIKey |
GZOVJNIKHQXVIU-FDJWOJMMSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | LOX-5 messenger RNA (ALOX5 mRNA) | Target Info | Inhibitor | [1] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Arachidonic acid metabolism | |||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Substituted coumarins as potent 5-lipoxygenase inhibitors. Bioorg Med Chem Lett. 2006 May 1;16(9):2528-31. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

