Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06AJN
|
|||
| Former ID |
DIB019561
|
|||
| Drug Name |
PMID7629799C6
|
|||
| Synonyms |
compound 3 [PMID: 8576905]; GTPL3063; BDBM50292846
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1], [2] | |
| Structure |
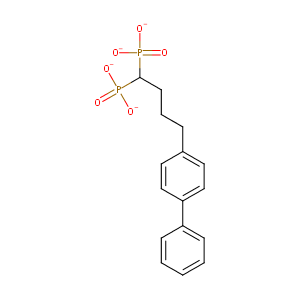 |
Download2D MOL
|
||
| Formula |
C16H16O6P2-4
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)C2=CC=C(C=C2)CCCC(P(=O)([O-])[O-])P(=O)([O-])[O-]
|
|||
| InChI |
1S/C16H20O6P2/c17-23(18,19)16(24(20,21)22)8-4-5-13-9-11-15(12-10-13)14-6-2-1-3-7-14/h1-3,6-7,9-12,16H,4-5,8H2,(H2,17,18,19)(H2,20,21,22)/p-4
|
|||
| InChIKey |
UQVFFWAGEQWWMP-UHFFFAOYSA-J
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Squalene synthetase (FDFT1) | Target Info | Inhibitor | [3] |
| BioCyc | Cholesterol biosynthesis II (via 24,25-dihydrolanosterol) | |||
| Cholesterol biosynthesis III (via desmosterol) | ||||
| Cholesterol biosynthesis I | ||||
| Superpathway of cholesterol biosynthesis | ||||
| Epoxysqualene biosynthesis | ||||
| KEGG Pathway | Steroid biosynthesis | |||
| Metabolic pathways | ||||
| Biosynthesis of antibiotics | ||||
| Panther Pathway | Cholesterol biosynthesis | |||
| Pathwhiz Pathway | Steroid Biosynthesis | |||
| Reactome | Cholesterol biosynthesis | |||
| PPARA activates gene expression | ||||
| Activation of gene expression by SREBF (SREBP) | ||||
| WikiPathways | Statin Pathway | |||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | ||||
| Activation of Gene Expression by SREBP (SREBF) | ||||
| SREBP signalling | ||||
| Cholesterol Biosynthesis | ||||
| Cholesterol biosynthesis | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 1,1-Bisphosphonate squalene synthase inhibitors: interplay between the isoprenoid subunit and the diphosphate surrogate. J Med Chem. 1995 Jul 7;38(14):2596-605. | |||
| REF 2 | Alpha-Phosphonosulfonic acids: potent and selective inhibitors of squalene synthase. J Med Chem. 1996 Feb 2;39(3):657-60. | |||
| REF 3 | Phosphonosulfonates are potent, selective inhibitors of dehydrosqualene synthase and staphyloxanthin biosynthesis in Staphylococcus aureus. J Med Chem. 2009 Feb 26;52(4):976-88. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

