Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05TOJ
|
|||
| Former ID |
DIB010843
|
|||
| Drug Name |
Peretinoin
|
|||
| Synonyms |
Polyprenoic acid; E-5166; NIK-333; Peretinoin (oral); Peretinoin (oral), Kowa; Peretinoin (oral formulation, hepatocellular carcinoma), Kowa Pharmaceutical
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hepatocellular carcinoma [ICD-11: 2C12.02; ICD-10: C22.0; ICD-9: 155] | Phase 3 | [1] | |
| Company |
Kowa Pharmaceutical Co Ltd; Eisai Co Ltd
|
|||
| Structure |
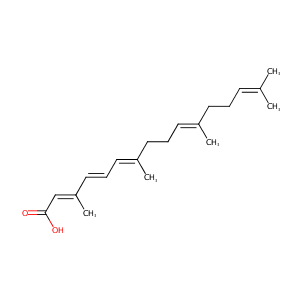 |
Download2D MOL |
||
| Formula |
C20H30O2
|
|||
| Canonical SMILES |
CC(=CCCC(=CCCC(=CC=CC(=CC(=O)O)C)C)C)C
|
|||
| InChI |
1S/C20H30O2/c1-16(2)9-6-10-17(3)11-7-12-18(4)13-8-14-19(5)15-20(21)22/h8-9,11,13-15H,6-7,10,12H2,1-5H3,(H,21,22)/b14-8+,17-11+,18-13+,19-15+
|
|||
| InChIKey |
UUBHZHZSIKRVIV-KCXSXWJSSA-N
|
|||
| CAS Number |
CAS 81485-25-8
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Retinoic acid receptor (RAR) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01640808) Study of Peretinoin for Suppressing Recurrence of HCV-positive HCC. U.S. National Institutes of Health. | |||
| REF 2 | Peretinoin, an acyclic retinoid, improves the hepatic gene signature of chronic hepatitis C following curative therapy of hepatocellular carcinoma. BMC Cancer. 2013 Apr 15;13:191. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

