Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05SSC
|
|||
| Former ID |
DCL000513
|
|||
| Drug Name |
Dalcetrapib
|
|||
| Synonyms |
JTT 705; JTT-705; R-1658; RG-1658; RO-4607381; S-[2-[[1-(2-ethylbutyl)cyclohexanecarbonyl]amino]phenyl] 2-methylpropanethioate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute coronary syndrome [ICD-11: BA41; ICD-9: 411.1] | Phase 3 | [1] | |
| Hyperlipidaemia [ICD-11: 5C80; ICD-10: E78] | Phase 3 | [2] | ||
| Company |
Roche
|
|||
| Structure |
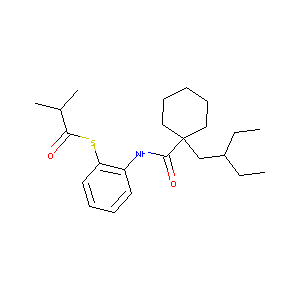 |
Download2D MOL |
||
| Formula |
C23H35NO2S
|
|||
| Canonical SMILES |
CCC(CC)CC1(CCCCC1)C(=O)NC2=CC=CC=C2SC(=O)C(C)C
|
|||
| InChI |
1S/C23H35NO2S/c1-5-18(6-2)16-23(14-10-7-11-15-23)22(26)24-19-12-8-9-13-20(19)27-21(25)17(3)4/h8-9,12-13,17-18H,5-7,10-11,14-16H2,1-4H3,(H,24,26)
|
|||
| InChIKey |
YZQLWPMZQVHJED-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 211513-37-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
12015448, 14927643, 17194949, 43529910, 57371967, 79689040, 103294369, 103990387, 114788136, 124490448, 126671653, 126731316, 129871265, 134339100, 135261049, 136367976, 137157003, 142130542, 143495520, 144115880, 144221104, 152106359, 152159578, 152234923, 152258615, 160647450, 162011639, 162205179, 162221183, 163914131, 164194108, 165246672, 170498253, 174528320, 177748646, 180387047, 185972146, 198984686, 198991964, 223471416, 223664086, 226840175, 241051420, 251971148, 252160607, 252215631, 252314519
|
|||
| ChEBI ID |
CHEBI:95001
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cholesteryl ester transfer protein (CETP) | Target Info | Inhibitor | [3] |
| Panther Pathway | CCKR signaling map ST | |||
| Reactome | LDL-mediated lipid transport | |||
| HDL-mediated lipid transport | ||||
| WikiPathways | Statin Pathway | |||
| Lipid digestion, mobilization, and transport | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | ClinicalTrials.gov (NCT00658515) A Study of RO4607381 in Stable Coronary Heart Disease Patients With Recent Acute Coronary Syndrome. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of Roche (2009). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

