Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05QOR
|
|||
| Former ID |
DNC013258
|
|||
| Drug Name |
SCOPOLETIN
|
|||
| Synonyms |
scopoletin; 92-61-5; Gelseminic acid; 6-Methylesculetin; 7-Hydroxy-6-methoxy-2H-chromen-2-one; Chrysatropic acid; Scopoletine; 7-Hydroxy-6-methoxycoumarin; Scopoletol; Murrayetin; 6-O-Methylesculetin; Escopoletin; 6-Methoxy-7-hydroxycoumarin; 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one; 6-Methoxyumbelliferone; 2H-1-Benzopyran-2-one, 7-hydroxy-6-methoxy-; Esculetin 6-methyl ether; beta-Methylesculetin; Esculetin-6-methyl ether; .beta.-Methylesculetin; Buxuletin; UNII-KLF1HS0SXJ; COUMARIN, 7-HYDROXY-6-METHOXY-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
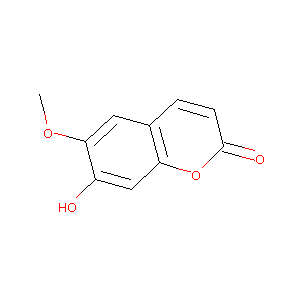 |
Download2D MOL |
||
| Formula |
C10H8O4
|
|||
| Canonical SMILES |
COC1=C(C=C2C(=C1)C=CC(=O)O2)O
|
|||
| InChI |
1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3
|
|||
| InChIKey |
RODXRVNMMDRFIK-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 92-61-5
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:17488
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 15-lipoxygenase (15-LOX) | Target Info | Inhibitor | [1] |
| BioCyc | Resolvin D biosynthesis | |||
| Lipoxin biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Linoleic acid metabolism | ||||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Panther Pathway | Inflammation mediated by chemokine and cytokine signaling pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| Pathway Interaction Database | IL4-mediated signaling events | |||
| WikiPathways | Arachidonic acid metabolism | |||
| Eicosanoid Synthesis | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Lipoxygenase inhibitory constituents of the fruits of noni (Morinda citrifolia) collected in Tahiti. J Nat Prod. 2007 May;70(5):859-62. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

