Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04YHR
|
|||
| Drug Name |
Samarium Sm-153 Lexidronam Pentasodium
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Cancer related pain [ICD-11: MG30; ICD-10: R52, G89] | Approved | [1] | |
| Therapeutic Class |
Neurology Agents
|
|||
| Company |
Lantheus Medical Imaging Inc
|
|||
| Structure |
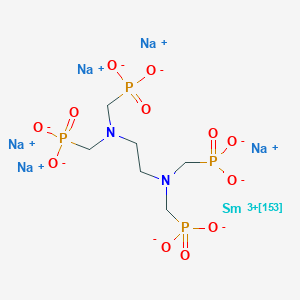 |
Download2D MOL
|
||
| Formula |
C6H12N2Na5O12P4Sm
|
|||
| Canonical SMILES |
C(CN(CP(=O)([O-])[O-])CP(=O)([O-])[O-])N(CP(=O)([O-])[O-])CP(=O)([O-])[O-].[Na+].[Na+].[Na+].[Na+].[Na+].[Sm+3]
|
|||
| InChI |
1S/C6H20N2O12P4.5Na.Sm/c9-21(10,11)3-7(4-22(12,13)14)1-2-8(5-23(15,16)17)6-24(18,19)20;;;;;;/h1-6H2,(H2,9,10,11)(H2,12,13,14)(H2,15,16,17)(H2,18,19,20);;;;;;/q;5*+1;+3/p-8/i;;;;;;1+3
|
|||
| InChIKey |
SZZACTGRBZTAKY-NKNBZPHVSA-F
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Samarium (SAM) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

