Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04TQO
|
|||
| Former ID |
DNCL003361
|
|||
| Drug Name |
Tenapanor
|
|||
| Synonyms |
1234423-95-0; UNII-WYD79216A6; AZD 1722; WYD79216A6; AZD-1722; 3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)-N-(26-((3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)phenyl)sulfonamido)-10,17-dioxo-3,6,21,24-tetraoxa-9,11,16,18-tetraazahexacosyl)benzenesulfonamide; Tenapanor [USAN:INN]; Tenapanor(free base); GTPL8449; CHEMBL3304485; SCHEMBL15267600; DTXSID40154016; BCP24892; BCP28554; EX-A2506
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Irritable bowel syndrome [ICD-11: DD91.0; ICD-10: K55-K64, K58; ICD-9: 564.1, 787.91] | Approved | [1] | |
| Constipation [ICD-11: DD91.1; ICD-10: K59.0; ICD-9: 564] | Phase 2 | [2] | ||
| Kidney disease [ICD-11: GC2Z] | Phase 2 | [3] | ||
| Company |
AstraZeneca
|
|||
| Structure |
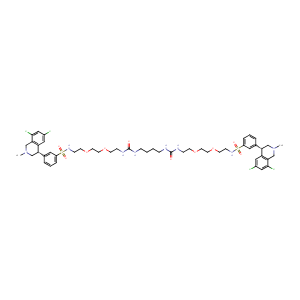 |
Download2D MOL
|
||
| Formula |
C50H66Cl4N8O10S2
|
|||
| Canonical SMILES |
CN1CC(C2=C(C1)C(=CC(=C2)Cl)Cl)C3=CC(=CC=C3)S(=O)(=O)NCCOCCOCCNC(=O)NCCCCNC(=O)NCCOCCOCCNS(=O)(=O)C4=CC=CC(=C4)C5CN(CC6=C5C=C(C=C6Cl)Cl)C
|
|||
| InChI |
1S/C50H66Cl4N8O10S2/c1-61-31-43(41-27-37(51)29-47(53)45(41)33-61)35-7-5-9-39(25-35)73(65,66)59-15-19-71-23-21-69-17-13-57-49(63)55-11-3-4-12-56-50(64)58-14-18-70-22-24-72-20-16-60-74(67,68)40-10-6-8-36(26-40)44-32-62(2)34-46-42(44)28-38(52)30-48(46)54/h5-10,25-30,43-44,59-60H,3-4,11-24,31-34H2,1-2H3,(H2,55,57,63)(H2,56,58,64)/t43-,44-/m0/s1
|
|||
| InChIKey |
DNHPDWGIXIMXSA-CXNSMIOJSA-N
|
|||
| CAS Number |
CAS 1234423-95-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Sodium/hydrogen exchanger 3 (SLC9A3) | Target Info | Modulator | [1] |
| KEGG Pathway | Proximal tubule bicarbonate reclamation | |||
| Protein digestion and absorption | ||||
| Bile secretion | ||||
| Mineral absorption | ||||
| Pathway Interaction Database | Endothelins | |||
| RhoA signaling pathway | ||||
| WikiPathways | SIDS Susceptibility Pathways | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | |||
| REF 2 | ClinicalTrials.gov (NCT01340053) A Study to Evaluate the Safety and Efficacy of RDX5791 for the Treatment of Constipation Predominant Irritable Bowel Syndrome (IBS-C). U.S. National Institutes of Health. | |||
| REF 3 | ClinicalTrials.gov (NCT01764854) Pharmacodynamic Study of AZD1722 in End-stage Renal Disease Patients on Hemodialysis. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

