Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04OXY
|
|||
| Former ID |
DNC008341
|
|||
| Drug Name |
2-methoxy-1,4-naphthoquinone
|
|||
| Synonyms |
2-Methoxy-1,4-naphthoquinone; 2348-82-5; 2-Methoxynaphthoquinone; 2-methoxynaphthalene-1,4-dione; 2-Methoxy-p-naphthoquinone; 1,4-Naphthalenedione, 2-methoxy-; 1,4-NAPHTHOQUINONE, 2-METHOXY-; UNII-39020BUT1D; 2-Methoxy-1,4-naphthalenedione; NSC 31530; AI3-17893; CHEMBL106562; 2-Methoxy-[1,4]naphthoquinone; CHEBI:69522; OBGBGHKYJAOXRR-UHFFFAOYSA-N; 39020BUT1D; 2-methoxy-1,4-dihydronaphthalene-1,4-dione; ACMC-20ans6; AC1L28UU; MLS002472995; 2-methoxy-1,4-napthoquinone; SCHEMBL571061; AC1Q483I; DTXSID1062338; CTK4F1565
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
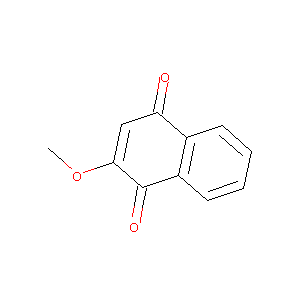 |
Download2D MOL |
||
| Formula |
C11H8O3
|
|||
| Canonical SMILES |
COC1=CC(=O)C2=CC=CC=C2C1=O
|
|||
| InChI |
1S/C11H8O3/c1-14-10-6-9(12)7-4-2-3-5-8(7)11(10)13/h2-6H,1H3
|
|||
| InChIKey |
OBGBGHKYJAOXRR-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 2348-82-5
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:69522
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Indoleamine 2,3-dioxygenase 1 (IDO1) | Target Info | Inhibitor | [1] |
| BioCyc | Superpathway of tryptophan utilization | |||
| Tryptophan degradation | ||||
| L-kynurenine degradation | ||||
| Tryptophan degradation to 2-amino-3-carboxymuconate semialdehyde | ||||
| NAD de novo biosynthesis | ||||
| KEGG Pathway | Tryptophan metabolism | |||
| Metabolic pathways | ||||
| African trypanosomiasis | ||||
| NetPath Pathway | TSLP Signaling Pathway | |||
| IL5 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | ||||
| Pathwhiz Pathway | Tryptophan Metabolism | |||
| Reactome | Tryptophan catabolism | |||
| WikiPathways | Tryptophan metabolism | |||
| Metabolism of amino acids and derivatives | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Indoleamine 2,3-dioxygenase is the anticancer target for a novel series of potent naphthoquinone-based inhibitors. J Med Chem. 2008 Mar 27;51(6):1706-18. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

