Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04HQJ
|
|||
| Former ID |
DIB012148
|
|||
| Drug Name |
AB-103
|
|||
| Synonyms |
Superantigen toxin antagonist (iv/infusion, septic shock/toxic shock), Atox Bio/Hebrew University of Jerusalem
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Necrotizing soft tissue infection [ICD-11: 1B71; ICD-10: M72.6] | Phase 3 | [1] | |
| Company |
Atoxbio; fast-track drugs & biologics
|
|||
| Structure |
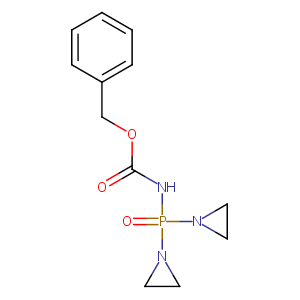 |
Download2D MOL |
||
| Formula |
C12H16N3O3P
|
|||
| Canonical SMILES |
C1CN1P(=O)(NC(=O)OCC2=CC=CC=C2)N3CC3
|
|||
| InChI |
1S/C12H16N3O3P/c16-12(18-10-11-4-2-1-3-5-11)13-19(17,14-6-7-14)15-8-9-15/h1-5H,6-10H2,(H,13,16,17)
|
|||
| InChIKey |
VFIUCBTYGKMLCM-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1980-45-6
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | T-cell-specific surface glycoprotein CD28 (CD28) | Target Info | Antagonist | [2], [3] |
| KEGG Pathway | Cell adhesion molecules (CAMs) | |||
| T cell receptor signaling pathway | ||||
| Intestinal immune network for IgA production | ||||
| Type I diabetes mellitus | ||||
| Measles | ||||
| Autoimmune thyroid disease | ||||
| Systemic lupus erythematosus | ||||
| Rheumatoid arthritis | ||||
| Allograft rejection | ||||
| Graft-versus-host disease | ||||
| Viral myocarditis | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | T cell activation | |||
| Pathway Interaction Database | TCR signaling in naï | |||
| TCR signaling in naï | ||||
| IL12 signaling mediated by STAT4 | ||||
| Reactome | PIP3 activates AKT signaling | |||
| Constitutive Signaling by Aberrant PI3K in Cancer | ||||
| CD28 co-stimulation | ||||
| CD28 dependent PI3K/Akt signaling | ||||
| CD28 dependent Vav1 pathway | ||||
| WikiPathways | TCR Signaling Pathway | |||
| Inflammatory Response Pathway | ||||
| Host Interactions of HIV factors | ||||
| PIP3 activates AKT signaling | ||||
| T-Cell Receptor and Co-stimulatory Signaling | ||||
| Allograft Rejection | ||||
| Costimulation by the CD28 family | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02469857) Phase III Efficacy and Safety Study of AB103 in the Treatment of Patients With Necrotizing Soft Tissue Infections. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

