Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03SHX
|
|||
| Former ID |
DNC006927
|
|||
| Drug Name |
5-iodo,5'-deoxytubercidin
|
|||
| Synonyms |
CHEMBL66280; 7-(5-Deoxy-Beta-D-Ribofuranosyl)-5-Iodo-7h-Pyrrolo[2,3-D]pyrimidin-4-Amine; 5-iodo,5'-deoxytubercidin; 2i6a; 5-Iodo-5-deoxytubercidin; 5-Iodo-5'-deoxytubercidin; SCHEMBL6236636; BDBM14486; DB07173; 5I5; (2R,3R,4S,5R)-2-{4-amino-5-iodopyrrolo[2,3-d]pyrimidin-7-yl}-5-methyloxolane-3,4-diol; 5'-DEOXY-5-IODOTUBERCIDIN; (2R,3R,4S,5R)-2-(4-AMINO-5-IODO-7H-PYRROLO[2,3-D]PYRIMIDIN-7-YL)-5-(METHYL)TETRAHYDROFURAN-3,4-DIOL; (2R,3R,4S,5R)-2-{4-amino-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-7-yl}-5-methyloxolane-3,4-diol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
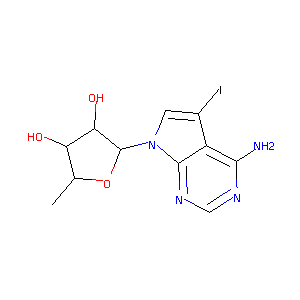 |
Download2D MOL |
||
| Formula |
C11H13IN4O3
|
|||
| Canonical SMILES |
CC1C(C(C(O1)N2C=C(C3=C(N=CN=C32)N)I)O)O
|
|||
| InChI |
1S/C11H13IN4O3/c1-4-7(17)8(18)11(19-4)16-2-5(12)6-9(13)14-3-15-10(6)16/h2-4,7-8,11,17-18H,1H3,(H2,13,14,15)/t4-,7-,8-,11-/m1/s1
|
|||
| InChIKey |
NTXUAWGNGBSCRS-TZQXKBMNSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adenosine kinase (ADK) | Target Info | Inhibitor | [1] |
| BioCyc | Superpathway of purine nucleotide salvage | |||
| Adenine and adenosine salvage II | ||||
| KEGG Pathway | Purine metabolism | |||
| Metabolic pathways | ||||
| Reactome | Purine salvage | |||
| WikiPathways | Metabolism of nucleotides | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Crystal structures of human adenosine kinase inhibitor complexes reveal two distinct binding modes. J Med Chem. 2006 Nov 16;49(23):6726-31. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

