Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03QJL
|
|||
| Former ID |
DCL000838
|
|||
| Drug Name |
Ibudilast
|
|||
| Synonyms |
Eyevinal; Ibudilastum; Ketas; Ibudilastum [Latin]; Ke Tas; AV 411; I 0157; KC 404; AV-411; Ibudilast [INN:JAN]; KC-404; Ketas (TN); MN-166; Ibudilast (JAN/INN); 1-(2-Isopropylpyrazolo(1,5-a)pyridin-3-yl)-2-methyl-1-propanone; 2-Isopropyl-3-isobutyrylpyrazolo(1,5-a)pyridine; 2-Methyl-1-[2-(1-methylethyl)pyrazolo[1,5-a]pyridin-3-yl] 1-propanone; 2-methyl-1-(2-propan-2-ylpyrazolo[1,5-a]pyridin-3-yl)propan-1-one; 3-Isobutyryl-2-isopropylpyrazolo(1,5-a)pyridine; 3-Isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Castleman's disease [ICD-11: 4B2Y] | Approved | [1] | |
| Alcohol dependence [ICD-11: 6C40.2; ICD-10: F10.2; ICD-9: 303] | Phase 2 | [2] | ||
| Amyotrophic lateral sclerosis [ICD-11: 8B60.0] | Phase 2 | [3] | ||
| Methamphetamine dependence [ICD-11: 6C46.2; ICD-10: F15.2] | Phase 2 | [2] | ||
| Multiple sclerosis [ICD-11: 8A40; ICD-9: 340] | Phase 2 | [3], [4] | ||
| Opioid dependence [ICD-11: 6C43.2Z] | Phase 2 | [2] | ||
| Company |
Avigen
|
|||
| Structure |
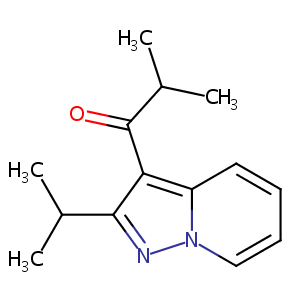 |
Download2D MOL |
||
| Formula |
C14H18N2O
|
|||
| Canonical SMILES |
CC(C)C1=NN2C=CC=CC2=C1C(=O)C(C)C
|
|||
| InChI |
1S/C14H18N2O/c1-9(2)13-12(14(17)10(3)4)11-7-5-6-8-16(11)15-13/h5-10H,1-4H3
|
|||
| InChIKey |
ZJVFLBOZORBYFE-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 50847-11-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
6240817, 7848448, 8152317, 11111307, 11114182, 12013668, 14822917, 17405161, 24278482, 29222794, 46500478, 47440428, 50030118, 50104937, 50104938, 53777705, 53788639, 57321931, 58107098, 79769657, 81041136, 81092873, 85211314, 85231085, 85787627, 87561227, 90341511, 91613941, 92303863, 92308717, 92711901, 99443231, 103185681, 104098264, 104304184, 121361284, 124397894, 124749844, 124800500, 124880396, 124880397, 124880398, 124887049, 125351724, 126630415, 126657050, 126670483, 129913079, 131316644, 134339304
|
|||
| ChEBI ID |
CHEBI:31684
|
|||
| SuperDrug ATC ID |
R03DC04
|
|||
| SuperDrug CAS ID |
cas=050847115
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Phosphodiesterase 3 (PDE3) | Target Info | Inhibitor | [1] |
| Phosphodiesterase 4 (PDE4) | Target Info | Inhibitor | [1] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Therapeutic targeting of 3',5'-cyclic nucleotide phosphodiesterases: inhibition and beyond. Nat Rev Drug Discov. 2019 Oct;18(10):770-796. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

