Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03MFQ
|
|||
| Former ID |
DNCL001971
|
|||
| Drug Name |
CHF-5074
|
|||
| Synonyms |
CHF 5074; CHF5074; 749269-83-8; CHF-5074; Itanapraced; UNII-C35RF1MWQZ; GHF-5074; C35RF1MWQZ; CHEMBL196945; 1-(3',4'-dichloro-2-fluoro(1,1'-biphenyl)-4-yl)cyclopropanecarboxylic acid; 1-[4-(3,4-dichlorophenyl)-3-fluorophenyl]cyclopropane-1-carboxylic acid; 1-(3',4'-Dichloro-2-Fluorobiphenyl-4-Yl)cyclopropanecarboxylic Acid; H50; SCHEMBL407631; GTPL7339; DTXSID30225901; AOB5325; LIYLTQQDABRNRX-UHFFFAOYSA-N; ZINC3986651; EX-A1963; BDBM50172482; AKOS026750398; SB16945; CS-5022; NCGC00408905-01; AS-16850; HY-14399; BC600569; FT-0708261
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 2 | [1], [2] | |
| Cognitive impairment [ICD-11: 6D71; ICD-10: F06.7; ICD-9: 331.83] | Phase 2 | [3], [4] | ||
| Company |
Chiesi Pharmaceuticals
|
|||
| Structure |
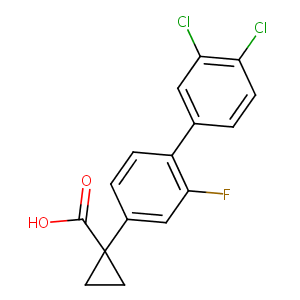 |
Download2D MOL |
||
| Formula |
C16H11Cl2FO2
|
|||
| Canonical SMILES |
C1CC1(C2=CC(=C(C=C2)C3=CC(=C(C=C3)Cl)Cl)F)C(=O)O
|
|||
| InChI |
1S/C16H11Cl2FO2/c17-12-4-1-9(7-13(12)18)11-3-2-10(8-14(11)19)16(5-6-16)15(20)21/h1-4,7-8H,5-6H2,(H,20,21)
|
|||
| InChIKey |
LIYLTQQDABRNRX-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 749269-83-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7339). | |||
| REF 2 | ClinicalTrials.gov (NCT01303744) Evaluation of Safety & Tolerability of Multiple Dose Regimens of CHF 5074 and Exploration of Effects on Potential Markers of Clinical Efficacy in Patients With Mild Cognitive Impairment. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 5 | CHF5074, a novel gamma-secretase modulator, attenuates brain beta-amyloid pathology and learning deficit in a mouse model of Alzheimer's disease.Br J Pharmacol.2009 Mar;156(6):982-93. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

