Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03LFR
|
|||
| Former ID |
DCL000392
|
|||
| Drug Name |
SB-756050
|
|||
| Indication | Type-2 diabetes [ICD-11: 5A11; ICD-9: 250] | Discontinued in Phase 2 | [1] | |
| Company |
GlaxoSmithKline
|
|||
| Structure |
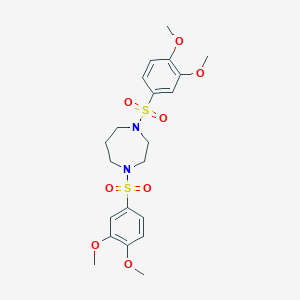 |
Download2D MOL |
||
| Formula |
C21H28N2O8S2
|
|||
| Canonical SMILES |
COC1=C(C=C(C=C1)S(=O)(=O)N2CCCN(CC2)S(=O)(=O)C3=CC(=C(C=C3)OC)OC)OC
|
|||
| InChI |
1S/C21H28N2O8S2/c1-28-18-8-6-16(14-20(18)30-3)32(24,25)22-10-5-11-23(13-12-22)33(26,27)17-7-9-19(29-2)21(15-17)31-4/h6-9,14-15H,5,10-13H2,1-4H3
|
|||
| InChIKey |
GJUFPAZNBPFNRI-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Farnesoid X-activated receptor (FXR) | Target Info | Agonist | [2] |
| KEGG Pathway | Bile secretion | |||
| Pathway Interaction Database | RXR and RAR heterodimerization with other nuclear receptor | |||
| Reactome | Recycling of bile acids and salts | |||
| PPARA activates gene expression | ||||
| Endogenous sterols | ||||
| WikiPathways | Nuclear Receptors in Lipid Metabolism and Toxicity | |||
| Nuclear Receptors Meta-Pathway | ||||
| Farnesoid X Receptor Pathway | ||||
| Drug Induction of Bile Acid Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800028284) | |||
| REF 2 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

