Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03AOP
|
|||
| Former ID |
DNCL003435
|
|||
| Drug Name |
DS-8500
|
|||
| Indication | Diabetic complication [ICD-11: 5A2Y; ICD-9: 253.5, 588.1] | Phase 2 | [1] | |
| Type-2 diabetes [ICD-11: 5A11; ICD-9: 250] | Phase 2 | [2] | ||
| Company |
Daiichi Sankyo
|
|||
| Structure |
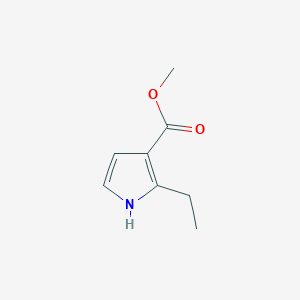 |
Download2D MOL |
||
| Formula |
C8H11NO2
|
|||
| Canonical SMILES |
CCC1=C(C=CN1)C(=O)OC
|
|||
| InChI |
1S/C8H11NO2/c1-3-7-6(4-5-9-7)8(10)11-2/h4-5,9H,3H2,1-2H3
|
|||
| InChIKey |
FWZOVQKKSCXOHE-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glucose-dependent insulinotropic receptor (GPR119) | Target Info | Modulator | [3] |
| KEGG Pathway | cAMP signaling pathway | |||
| Insulin secretion | ||||
| WikiPathways | Incretin Synthesis, Secretion, and Inactivation | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02222350) Phase 2 Study of DS-8500a in Patients With Type 2 Diabetes. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Addressing Unmet Medical Needs in Type 2 Diabetes: A Narrative Review of Drugs under Development. Curr Diabetes Rev. 2015 March; 11(1): 17-31. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

