Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02ZHW
|
|||
| Former ID |
DIB016950
|
|||
| Drug Name |
177Lu-DOTA-octreotate
|
|||
| Synonyms |
Lutate; Octreotate [177Lu]; Lutetium-177 octreotate; 177-Lu octreotate; 177-Lu-radiolabeled somatostatin receptor-targeted peptide (neuroendocrine tumor), Biosynthema; 177-lutetium-radiolabeled somatostatin receptor-targeted peptide (neuroendocrinetumor), Biosynthema; 177Lutetium DOTA0-Tyr3-Octreotate
Click to Show/Hide
|
|||
| Indication | Melanoma [ICD-11: 2C30; ICD-9: 172] | Phase 3 | [1] | |
| Neuroendocrine cancer [ICD-11: 2B72.1; ICD-9: 209] | Phase 3 | [2] | ||
| Company |
Advanced accelerator applications
|
|||
| Structure |
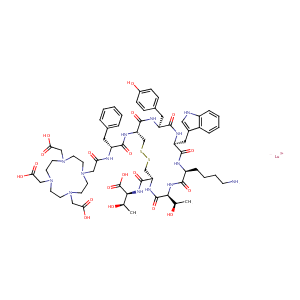 |
Download2D MOL |
||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Somatostatin receptor (SSTR) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01578239) A Study Comparing Treatment With 177Lu-DOTA0-Tyr3-Octreotate to Octreotide LAR in Patients With Inoperable, Progressive, Somatostatin Receptor Positive Midgut Carcinoid Tumours. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014 May;43(4):518-25. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

