Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02MRN
|
|||
| Former ID |
DIB003684
|
|||
| Drug Name |
Golotimod
|
|||
| Synonyms |
229305-39-9; SCV-07; gamma-D-Glu-L-trp; SCV07; gamma-D-Glutamyl-L-tryptophan; SCV 07; UNII-637C487Y09; 637C487Y09; (R)-2-Amino-5-(((S)-1-carboxy-2-(1H-indol-3-yl)ethyl)amino)-5-oxopentanoic acid; Golotimod [USAN:INN]; (2R)-2-amino-5-[[(1S)-1-carboxy-2-(1H-indol-3-yl)ethyl]amino]-5-oxopentanoic acid; (2R)-2-Amino-5-(((1S)-1-carboxy-2-(1H-indol-3-yl)ethyl)amino)-5-oxopentanoic acid; Golotimod (USAN/INN); GAMMA-D-GLU-TRP-OH; H-D-Glu(L-Trp-OH)-OH; SCHEMBL727944; (gamma-glutamyl-L-tryptophan); CHEMBL2103812; Golotimod (oral); Golotimod (oral), SciClone/Verta; SCV-07 (oral, tuberculosis), SciClone/Verta
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Immune System disease [ICD-11: 4A01-4B41] | Phase 2 | [1] | |
| Inflammation [ICD-11: 1A00-CA43.1] | Discontinued in Phase 2 | [2] | ||
| Company |
Verta Ltd; SciClone Pharmaceuticals
|
|||
| Structure |
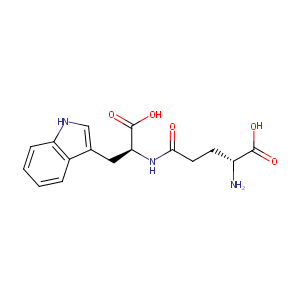 |
Download2D MOL |
||
| Formula |
C16H19N3O5
|
|||
| Canonical SMILES |
C1=CC=C2C(=C1)C(=CN2)CC(C(=O)O)NC(=O)CCC(C(=O)O)N
|
|||
| InChI |
1S/C16H19N3O5/c17-11(15(21)22)5-6-14(20)19-13(16(23)24)7-9-8-18-12-4-2-1-3-10(9)12/h1-4,8,11,13,18H,5-7,17H2,(H,19,20)(H,21,22)(H,23,24)/t11-,13+/m1/s1
|
|||
| InChIKey |
CATMPQFFVNKDEY-YPMHNXCESA-N
|
|||
| CAS Number |
CAS 229305-39-9
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00756951) Dose Ranging Study to Assess the Safety and Efficacy of SCV-07 for the Delay to Onset of Severe Oral Mucositis in Patients Receiving Chemoradiation Therapy for Head and Neck Cancer. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of SciClone. | |||
| REF 3 | National Cancer Institute Drug Dictionary (drug id 617379). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

