Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02JUT
|
|||
| Former ID |
DNC000149
|
|||
| Drug Name |
Acivicin
|
|||
| Synonyms |
acivicin; 42228-92-2; Antibiotic AT 125; Acivicinum; Acivicino; Acivicine; AT-125; AT 125; NSC-163501; NSC 163501; NSC163501; UNII-O0X60K76I6; ACIA; U 42126; CHEBI:74545; O0X60K76I6; U-42,126; Acivicin [USAN:INN]; (alphaS,5S)-alpha-Amino-3-chloro-2-isoxazoline-5-acetic acid; Acivicinum [INN-Latin]; Acivicine [INN-French]; Acivicino [INN-Spanish]; (alpha-S,5S)-alpha-Amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid; C5H7ClN2O3; (2S)-2-Amino-2-[(5S)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl]acetic acid; U-42126
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Structure |
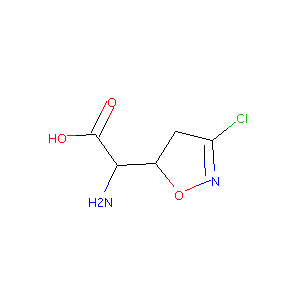 |
Download2D MOL |
||
| Formula |
C5H7ClN2O3
|
|||
| Canonical SMILES |
C1C(ON=C1Cl)C(C(=O)O)N
|
|||
| InChI |
1S/C5H7ClN2O3/c6-3-1-2(11-8-3)4(7)5(9)10/h2,4H,1,7H2,(H,9,10)/t2-,4-/m0/s1
|
|||
| InChIKey |
QAWIHIJWNYOLBE-OKKQSCSOSA-N
|
|||
| CAS Number |
CAS 42228-92-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
301209, 604683, 8137178, 8149785, 8874383, 11335847, 11361086, 11363237, 11365799, 11368361, 11372873, 11374503, 11376523, 11405636, 11446677, 11462058, 11491672, 11492717, 11494157, 12012692, 15171287, 17396913, 24769896, 26612246, 26679404, 34834567, 47440265, 47959748, 47959749, 50122781, 50927642, 53787108, 57401220, 85147462, 99301760, 104247289, 104495125, 124633291, 131314443, 134339664, 135000593, 137241165, 144205098, 162219966, 163132478, 163725839, 164784296, 179038041, 184573969, 198977092
|
|||
| ChEBI ID |
CHEBI:74545
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | CTP synthase (CTPS1) | Target Info | Inhibitor | [1], [2] |
| Leishmania Carbamoyl-phosphate synthase (Leishm CPS) | Target Info | Inhibitor | [3] | |
| KEGG Pathway | Pyrimidine metabolism | |||
| Metabolic pathways | ||||
| Panther Pathway | De novo pyrimidine ribonucleotides biosythesis | |||
| Pathwhiz Pathway | Pyrimidine Metabolism | |||
| WikiPathways | Metabolism of nucleotides | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Synergistic effects with inhibitors of de novo pyrimidine synthesis, acivicin, and N-(phosphonacetyl)-L-aspartic acid. Cancer Res. 1981 Sep;41(9 Pt 1):3419-23. | |||
| REF 2 | Trypanosoma brucei CTP synthetase: a target for the treatment of African sleeping sickness. Proc Natl Acad Sci U S A. 2001 May 22;98(11):6412-6. | |||
| REF 3 | Acivicin: a highly active potential chemotherapeutic agent against visceral leishmaniasis. Biochem Biophys Res Commun. 1990 Jul 31;170(2):426-32. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

