Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02JUJ
|
|||
| Former ID |
DNC008619
|
|||
| Drug Name |
5-(6-Methoxynaphthalen-2-yl)pyridin-3-ol
|
|||
| Synonyms |
CHEMBL500636; 5-(6-Methoxynaphthalen-2-yl)pyridin-3-ol; ZINC40425010
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
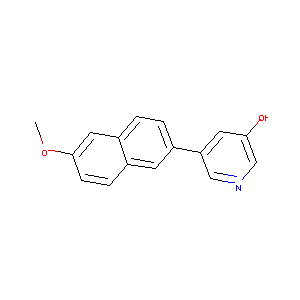 |
Download2D MOL |
||
| Formula |
C16H13NO2
|
|||
| Canonical SMILES |
COC1=CC2=C(C=C1)C=C(C=C2)C3=CC(=CN=C3)O
|
|||
| InChI |
1S/C16H13NO2/c1-19-16-5-4-11-6-12(2-3-13(11)8-16)14-7-15(18)10-17-9-14/h2-10,18H,1H3
|
|||
| InChIKey |
YXTBYYAJCILARD-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Steroid 11-beta-hydroxylase (CYP11B1) | Target Info | Inhibitor | [1] |
| BioCyc | Superpathway of steroid hormone biosynthesis | |||
| Glucocorticoid biosynthesis | ||||
| Mineralocorticoid biosynthesis | ||||
| KEGG Pathway | Steroid hormone biosynthesis | |||
| Metabolic pathways | ||||
| Pathwhiz Pathway | Steroidogenesis | |||
| Reactome | Glucocorticoid biosynthesis | |||
| Endogenous sterols | ||||
| WikiPathways | Metapathway biotransformation | |||
| Oxidation by Cytochrome P450 | ||||
| Metabolism of steroid hormones and vitamin D | ||||
| Corticotropin-releasing hormone | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Overcoming undesirable CYP1A2 inhibition of pyridylnaphthalene-type aldosterone synthase inhibitors: influence of heteroaryl derivatization on pote... J Med Chem. 2008 Aug 28;51(16):5064-74. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

